Insights into the Effects of Complement Factor H on the Assembly and Decay of the Alternative Pathway C3 Proconvertase and C3 Convertase
- PMID: 26903516
- PMCID: PMC4825022
- DOI: 10.1074/jbc.M115.693119
Insights into the Effects of Complement Factor H on the Assembly and Decay of the Alternative Pathway C3 Proconvertase and C3 Convertase
Retraction in
-
Withdrawals/Retractions: Insights into the effects of complement factor H on the assembly and decay of the alternative pathway C3 proconvertase and C3 convertase.J Biol Chem. 2017 Apr 14;292(15):6094. doi: 10.1074/jbc.A115.693119. J Biol Chem. 2017. PMID: 28411212 Free PMC article.
Abstract
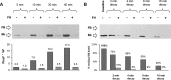
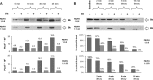
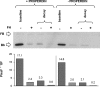
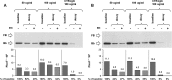
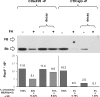
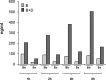
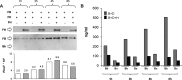
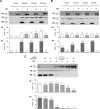
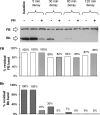
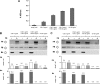
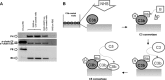
The activated fragment of C3 (C3b) and factor B form the C3 proconvertase (C3bB), which is cleaved by factor D to C3 convertase (C3bBb). Older studies (Conrad, D. H., Carlo, J. R., and Ruddy, S. (1978)J. Exp. Med.147, 1792-1805; Pangburn, M. K., and Müller-Eberhard, H. J. (1978)Proc. Natl. Acad. Sci. U.S.A.75, 2416-2420; Kazatchkine, M. D., Fearon, D. T., and Austen, K. F. (1979)J. Immunol.122, 75-81) indicated that the complement alternative pathway regulator factor H (FH) competes with factor B for C3b binding; however, the capability of FH to prevent C3bB assembly has not been formally investigated. Moreover, in the few published studies FH did not favor C3bB dissociation. Whether FH may affect C3bBb formation from C3bB is unknown. We set up user-friendly assays based on combined microplate/Western blotting techniques that specifically detect either C3bB or C3bBb, with the aim of investigating the effect of FH on C3bB assembly and decay and C3bBb formation and decay. We document that FH does not affect C3bB assembly, indicating that FH does not efficiently compete with factor B for C3b binding. We also found that FH does not dissociate C3bB. FH showed a strong C3bBb decay-accelerating activity, as reported previously, and also exerted an apparent inhibitory effect on C3bBb formation. The latter effect was not fully attributable to a rapid FH-mediated dissociation of C3bBb complexes, because blocking decay with properdin and C3 nephritic factor did not restore C3bBb formation. FH almost completely prevented release of the smaller cleavage subunit of FB (Ba), without modifying the amount of C3bB complexes, suggesting that FH inhibits the conversion of C3bB to C3bBb. Thus, the inhibitory effect of FH on C3bBb formation is likely the sum of inhibition of C3bB conversion to C3bBb and of C3bBb decay acceleration. Further studies are required to confirm these findings in physiological cell-based settings.
Keywords: C3 convertase; C3 nephritic factor; C3 proconvertase; Western blot; alternative pathway complement system; complement; convertase; factor H; magnesium; manganese.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

