Arabidopsis Small Rubber Particle Protein Homolog SRPs Play Dual Roles as Positive Factors for Tissue Growth and Development and in Drought Stress Responses
- PMID: 26903535
- PMCID: PMC4825120
- DOI: 10.1104/pp.16.00165
Arabidopsis Small Rubber Particle Protein Homolog SRPs Play Dual Roles as Positive Factors for Tissue Growth and Development and in Drought Stress Responses
Abstract
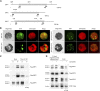
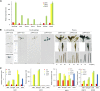
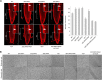
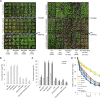
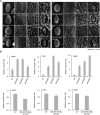
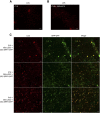
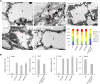
Lipid droplets (LDs) act as repositories for fatty acids and sterols, which are used for various cellular processes such as energy production and membrane and hormone synthesis. LD-associated proteins play important roles in seed development and germination, but their functions in postgermination growth are not well understood. Arabidopsis (Arabidopsis thaliana) contains three SRP homologs (SRP1, SRP2, and SRP3) that share sequence identities with small rubber particle proteins of the rubber tree (Hevea brasiliensis). In this report, the possible cellular roles of SRPs in postgermination growth and the drought tolerance response were investigated. Arabidopsis SRPs appeared to be LD-associated proteins and displayed polymerization properties in vivo and in vitro. SRP-overexpressing transgenic Arabidopsis plants (35S:SRP1, 35S:SRP2, and 35S:SRP3) exhibited higher vegetative and reproductive growth and markedly better tolerance to drought stress than wild-type Arabidopsis. In addition, constitutive over-expression of SRPs resulted in increased numbers of large LDs in postgermination seedlings. In contrast, single (srp1, 35S:SRP2-RNAi, and srp3) and triple (35S:SRP2-RNAi/srp1srp3) loss-of-function mutant lines exhibited the opposite phenotypes. Our results suggest that Arabidopsis SRPs play dual roles as positive factors in postgermination growth and the drought stress tolerance response. The possible relationships between LD-associated proteins and the drought stress response are discussed.
© 2016 American Society of Plant Biologists. All Rights Reserved.
Figures


Similar articles
-
The Arabidopsis RING E3 ubiquitin ligase AtAIRP2 plays combinatory roles with AtAIRP1 in abscisic acid-mediated drought stress responses.Plant Physiol. 2011 Dec;157(4):2240-57. doi: 10.1104/pp.111.185595. Epub 2011 Oct 10. Plant Physiol. 2011. PMID: 21969385 Free PMC article.
-
Overexpression of a wheat phospholipase D gene, TaPLDα, enhances tolerance to drought and osmotic stress in Arabidopsis thaliana.Planta. 2014 Jul;240(1):103-15. doi: 10.1007/s00425-014-2066-6. Epub 2014 Apr 5. Planta. 2014. PMID: 24705986
-
SKP1 is involved in abscisic acid signalling to regulate seed germination, stomatal opening and root growth in Arabidopsis thaliana.Plant Cell Environ. 2012 May;35(5):952-65. doi: 10.1111/j.1365-3040.2011.02464.x. Epub 2011 Dec 8. Plant Cell Environ. 2012. PMID: 22074111
-
Convergence and Divergence of Sugar and Cytokinin Signaling in Plant Development.Int J Mol Sci. 2021 Jan 28;22(3):1282. doi: 10.3390/ijms22031282. Int J Mol Sci. 2021. PMID: 33525430 Free PMC article. Review.
-
Inter-tissue and inter-organ signaling in drought stress response and phenotyping of drought tolerance.Plant J. 2022 Jan;109(2):342-358. doi: 10.1111/tpj.15619. Epub 2021 Dec 16. Plant J. 2022. PMID: 34863007 Free PMC article. Review.
Cited by
-
Plasticity of the Arabidopsis leaf lipidome and proteome in response to pathogen infection and heat stress.Plant Physiol. 2025 Feb 7;197(2):kiae274. doi: 10.1093/plphys/kiae274. Plant Physiol. 2025. PMID: 38781317 Free PMC article.
-
Response of high leaf-oil Arabidopsis thaliana plant lines to biotic or abiotic stress.Plant Signal Behav. 2018;13(5):e1464361. doi: 10.1080/15592324.2018.1464361. Epub 2018 Jun 4. Plant Signal Behav. 2018. PMID: 29701541 Free PMC article.
-
Lipid Body Dynamics in Shoot Meristems: Production, Enlargement, and Putative Organellar Interactions and Plasmodesmal Targeting.Front Plant Sci. 2021 Jul 21;12:674031. doi: 10.3389/fpls.2021.674031. eCollection 2021. Front Plant Sci. 2021. PMID: 34367200 Free PMC article.
-
Isolation of Lipid Droplets for Protein and Lipid Analysis.Methods Mol Biol. 2021;2295:295-320. doi: 10.1007/978-1-0716-1362-7_16. Methods Mol Biol. 2021. PMID: 34047983
-
The N-Terminal UND Motif of the Arabidopsis U-Box E3 Ligase PUB18 Is Critical for the Negative Regulation of ABA-Mediated Stomatal Movement and Determines Its Ubiquitination Specificity for Exocyst Subunit Exo70B1.Plant Cell. 2016 Dec;28(12):2952-2973. doi: 10.1105/tpc.16.00347. Epub 2016 Dec 12. Plant Cell. 2016. PMID: 27956469 Free PMC article.
References
-
- Atia A, Debez A, Barhoumi Z, Abdelly C, Smaoui A (2009) Histochemical localization of essential oils and bioactive substances in the seed coat of the halophyte Crithmum maritimum L. (Apiaceae). J Plant Biol 52: 448–452
-
- Aubert Y, Vile D, Pervent M, Aldon D, Ranty B, Simonneau T, Vavasseur A, Galaud JP (2010) RD20, a stress-inducible caleosin, participates in stomatal control, transpiration and drought tolerance in Arabidopsis thaliana. Plant Cell Physiol 51: 1975–1987 - PubMed
-
- Bae H, Choi SM, Yang SW, Pai H-S, Kim WT (2009) Suppression of the ER-localized AAA ATPase NgCDC48 inhibits tobacco growth and development. Mol Cells 28: 57–65 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

