Rhythmic expression of cryptochrome induces the circadian clock of arrhythmic suprachiasmatic nuclei through arginine vasopressin signaling
- PMID: 26903624
- PMCID: PMC4791030
- DOI: 10.1073/pnas.1519044113
Rhythmic expression of cryptochrome induces the circadian clock of arrhythmic suprachiasmatic nuclei through arginine vasopressin signaling
Abstract
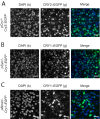
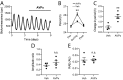
Circadian rhythms in mammals are coordinated by the suprachiasmatic nucleus (SCN). SCN neurons define circadian time using transcriptional/posttranslational feedback loops (TTFL) in which expression of Cryptochrome (Cry) and Period (Per) genes is inhibited by their protein products. Loss of Cry1 and Cry2 stops the SCN clock, whereas individual deletions accelerate and decelerate it, respectively. At the circuit level, neuronal interactions synchronize cellular TTFLs, creating a spatiotemporal wave of gene expression across the SCN that is lost in Cry1/2-deficient SCN. To interrogate the properties of CRY proteins required for circadian function, we expressed CRY in SCN of Cry-deficient mice using adeno-associated virus (AAV). Expression of CRY1::EGFP or CRY2::EGFP under a minimal Cry1 promoter was circadian and rapidly induced PER2-dependent bioluminescence rhythms in previously arrhythmic Cry1/2-deficient SCN, with periods appropriate to each isoform. CRY1::EGFP appropriately lengthened the behavioral period in Cry1-deficient mice. Thus, determination of specific circadian periods reflects properties of the respective proteins, independently of their phase of expression. Phase of CRY1::EGFP expression was critical, however, because constitutive or phase-delayed promoters failed to sustain coherent rhythms. At the circuit level, CRY1::EGFP induced the spatiotemporal wave of PER2 expression in Cry1/2-deficient SCN. This was dependent on the neuropeptide arginine vasopressin (AVP) because it was prevented by pharmacological blockade of AVP receptors. Thus, our genetic complementation assay reveals acute, protein-specific induction of cell-autonomous and network-level circadian rhythmicity in SCN never previously exposed to CRY. Specifically, Cry expression must be circadian and appropriately phased to support rhythms, and AVP receptor signaling is required to impose circuit-level circadian function.
Keywords: arginine vasopressin; bioluminescence; clock; oscillation; period.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Hastings MH, Reddy AB, Maywood ES. A clockwork web: Circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci. 2003;4(8):649–661. - PubMed
-
- van der Horst GT, et al. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature. 1999;398(6728):627–630. - PubMed
-
- Kume K, et al. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell. 1999;98(2):193–205. - PubMed
-
- Ukai-Tadenuma M, et al. Delay in feedback repression by cryptochrome 1 is required for circadian clock function. Cell. 2011;144(2):268–281. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

