Cellular Cholesterol Distribution Influences Proteolytic Release of the LRP-1 Ectodomain
- PMID: 26903870
- PMCID: PMC4751253
- DOI: 10.3389/fphar.2016.00025
Cellular Cholesterol Distribution Influences Proteolytic Release of the LRP-1 Ectodomain
Abstract
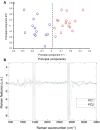
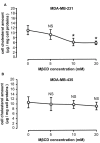
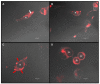
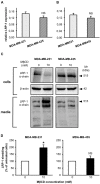
Low-density lipoprotein receptor-related protein-1 (LRP-1) is a multifunctional matricellular receptor composed of a large ligand-binding subunit (515-kDa α-chain) associated with a short trans-membrane subunit (85-kDa β-chain). LRP-1, which exhibits both endocytosis and cell signaling properties, plays a key role in tumor invasion by regulating the activity of proteinases such as matrix metalloproteinases (MMPs). LRP-1 is shed at the cell surface by proteinases such as membrane-type 1 MMP (MT1-MMP) and a disintegrin and metalloproteinase-12 (ADAM-12). Here, we show by using biophysical, biochemical, and cellular imaging approaches that efficient extraction of cell cholesterol and increased LRP-1 shedding occur in MDA-MB-231 breast cancer cells but not in MDA-MB-435 cells. Our data show that cholesterol is differently distributed in both cell lines; predominantly intracellularly for MDA-MB-231 cells and at the plasma membrane for MDA-MB-435 cells. This study highlights the relationship between the rate and cellular distribution of cholesterol and its impact on LRP-1 shedding modulation. Altogether, our data strongly suggest that the increase of LRP-1 shedding upon cholesterol depletion induces a higher accessibility of the sheddase substrate, i.e., LRP-1, at the cell surface rather than an increase of expression of the enzyme.
Keywords: LRP-1; Raman microspectroscopy; cholesterol; ectodomain; low-density lipoprotein receptor-related protein-1; shedding.
Figures
References
-
- Abramczyk H., Surmacki J., Kopeć M., Olejnik A. K., Lubecka-Pietruszewska K., Fabianowska-Majewska K. (2015). The role of lipid droplets and adipocytes in cancer. Raman imaging of cell cultures: MCF10A, MCF7, and MDA-MB-231 compared to adipocytes in cancerous human breast tissue. Analyst 140 2224–2235. 10.1039/c4an01875c - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

