New Bio-Indicators for Long Term Natural Attenuation of Monoaromatic Compounds in Deep Terrestrial Aquifers
- PMID: 26904000
- PMCID: PMC4746249
- DOI: 10.3389/fmicb.2016.00122
New Bio-Indicators for Long Term Natural Attenuation of Monoaromatic Compounds in Deep Terrestrial Aquifers
Abstract
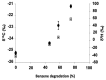
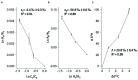
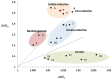
Deep subsurface aquifers despite difficult access, represent important water resources and, at the same time, are key locations for subsurface engineering activities for the oil and gas industries, geothermal energy, and CO2 or energy storage. Formation water originating from a 760 m-deep geological gas storage aquifer was sampled and microcosms were set up to test the biodegradation potential of BTEX by indigenous microorganisms. The microbial community diversity was studied using molecular approaches based on 16S rRNA genes. After a long incubation period, with several subcultures, a sulfate-reducing consortium composed of only two Desulfotomaculum populations was observed able to degrade benzene, toluene, and ethylbenzene, extending the number of hydrocarbonoclastic-related species among the Desulfotomaculum genus. Furthermore, we were able to couple specific carbon and hydrogen isotopic fractionation during benzene removal and the results obtained by dual compound specific isotope analysis (𝜀C = -2.4‰ ± 0.3‰; 𝜀H = -57‰ ± 0.98‰; AKIEC: 1.0146 ± 0.0009, and AKIEH: 1.5184 ± 0.0283) were close to those obtained previously in sulfate-reducing conditions: this finding could confirm the existence of a common enzymatic reaction involving sulfate-reducers to activate benzene anaerobically. Although we cannot assign the role of each population of Desulfotomaculum in the mono-aromatic hydrocarbon degradation, this study suggests an important role of the genus Desulfotomaculum as potential biodegrader among indigenous populations in subsurface habitats. This community represents the simplest model of benzene-degrading anaerobes originating from the deepest subterranean settings ever described. As Desulfotomaculum species are often encountered in subsurface environments, this study provides some interesting results for assessing the natural response of these specific hydrologic systems in response to BTEX contamination during remediation projects.
Keywords: BTEX; Desulfotomaculum; deep aquifer; natural attenuation; sulfate-reduction.
Figures



References
-
- Ahad J. M. E., Sherwood Lollar B., Edwards E. A., Slater G. F., Sleep B. E. (2000). Carbon isotope fractionation during anaerobic biodegradation of toluene: implications for intrinsic bioremediation. Environ. Sci. Technol. 34 892–896. 10.1021/es990797y - DOI
-
- Anderson R. T., Lovley D. R. (2000). Anaerobic bioremediation of benzene under sulfate-reducing conditions in a petroleum-contaminated aquifer. Environ. Sci. Technol. 34 2261–2266. 10.1021/es991211a - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

