Immunoprotective Efficacy of Acinetobacter baumannii Outer Membrane Protein, FilF, Predicted In silico as a Potential Vaccine Candidate
- PMID: 26904021
- PMCID: PMC4751259
- DOI: 10.3389/fmicb.2016.00158
Immunoprotective Efficacy of Acinetobacter baumannii Outer Membrane Protein, FilF, Predicted In silico as a Potential Vaccine Candidate
Abstract
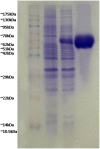
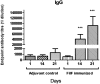
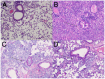
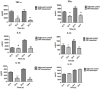
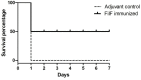
Acinetobacter baumannii is emerging as a serious nosocomial pathogen with multidrug resistance that has made it difficult to cure and development of efficacious treatment against this pathogen is direly needed. This has led to investigate vaccine approach to prevent and treat A. baumannii infections. In this work, an outer membrane putative pilus assembly protein, FilF, was predicted as vaccine candidate by in silico analysis of A. baumannii proteome and was found to be conserved among the A. baumannii strains. It was cloned and expressed in E. coli BL21(DE3) and purified by Ni-NTA chromatography. Immunization with FilF generated high antibody titer (>64,000) and provided 50% protection against a standardized lethal dose (10(8) CFU) of A. baumannii in murine pneumonia model. FilF immunization reduced the bacterial load in lungs by 2 and 4 log cycles, 12 and 24 h post infection as compared to adjuvant control; reduced the levels of pro-inflammatory cytokines TNF-α, IL-6, IL-33, IFN-γ, and IL-1β significantly and histology of lung tissue supported the data by showing considerably reduced damage and infiltration of neutrophils in lungs. These results demonstrate the in vivo validation of immunoprotective efficacy of a protein predicted as a vaccine candidate by in silico proteomic analysis and open the possibilities for exploration of a large array of uncharacterized proteins.
Keywords: Acinetobacter baumannii; FilF; OMP; cytokines; immunoprotection; reverse vaccinology; vaccine.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources

