Clearance Deficiency and Cell Death Pathways: A Model for the Pathogenesis of SLE
- PMID: 26904025
- PMCID: PMC4745266
- DOI: 10.3389/fimmu.2016.00035
Clearance Deficiency and Cell Death Pathways: A Model for the Pathogenesis of SLE
Abstract
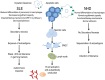
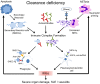
Alterations of cell death pathways, including apoptosis and the neutrophil specific kind of death called NETosis, can represent a potential source of autoantigens. Defects in the clearance of apoptotic cells may be responsible for the initiation of systemic autoimmunity in several chronic inflammatory diseases, including systemic lupus erythematosus (SLE). Autoantigens are released mainly from secondary necrotic cells because of a defective clearance of apoptotic cells or an inefficient degradation of DNA-containing neutrophil extracellular traps (NETs). These modified autoantigens are presented by follicular dendritic cells to autoreactive B cells in germinal centers of secondary lymphoid organs. This results in the loss of self-tolerance and production of autoantibodies, a unifying feature of SLE. Immune complexes (IC) are formed from autoantibodies bound to uncleared cellular debris in blood or tissues. Clearance of IC by blood phagocytes, macrophages, and dendritic cells leads to proinflammatory cytokine secretion. In particular, plasmacytoid dendritic cells produce high amounts of interferon-α upon IC uptake, thereby contributing to the interferon signature of patients with SLE. The clearance of antinuclear IC via Fc-gamma receptors is considered a central event in amplifying inflammatory immune responses in SLE. Along with this, the accumulation of cell remnants represents an initiating event of the etiology, while the subsequent generation of autoantibodies against nuclear antigens (including NETs) results in the perpetuation of inflammation and tissue damage in patients with SLE. Here, we discuss the implications of defective clearance of apoptotic cells and NETs in the development of clinical manifestations in SLE.
Keywords: NETosis; SLE; apoptosis; autoantibody; clearance.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

