Hypothesis: Paralog Formation from Progenitor Proteins and Paralog Mutagenesis Spur the Rapid Evolution of Telomere Binding Proteins
- PMID: 26904098
- PMCID: PMC4748036
- DOI: 10.3389/fgene.2016.00010
Hypothesis: Paralog Formation from Progenitor Proteins and Paralog Mutagenesis Spur the Rapid Evolution of Telomere Binding Proteins
Abstract
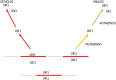
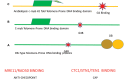
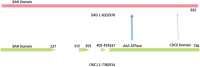
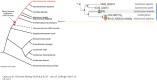
Through elegant studies in fungal cells and complex organisms, we propose a unifying paradigm for the rapid evolution of telomere binding proteins (TBPs) that associate with either (or both) telomeric DNA and telomeric proteins. TBPs protect and regulate telomere structure and function. Four critical factors are involved. First, TBPs that commonly bind to telomeric DNA include the c-Myb binding proteins, OB-fold single-stranded binding proteins, and G-G base paired Hoogsteen structure (G4) binding proteins. Each contributes independently or, in some cases, cooperatively, to provide a minimum level of telomere function. As a result of these minimal requirements and the great abundance of homologs of these motifs in the proteome, DNA telomere-binding activity may be generated more easily than expected. Second, telomere dysfunction gives rise to genome instability, through the elevation of recombination rates, genome ploidy, and the frequency of gene mutations. The formation of paralogs that diverge from their progenitor proteins ultimately can form a high frequency of altered TBPs with altered functions. Third, TBPs that assemble into complexes (e.g., mammalian shelterin) derive benefits from the novel emergent functions. Fourth, a limiting factor in the evolution of TBP complexes is the formation of mutually compatible interaction surfaces amongst the TBPs. These factors may have different degrees of importance in the evolution of different phyla, illustrated by the apparently simpler telomeres in complex plants. Selective pressures that can utilize the mechanisms of paralog formation and mutagenesis to drive TBP evolution along routes dependent on the requisite physiologic changes.
Keywords: evolution; models; non-LTR reverse transcription; stress response; telomerase; telomeres.
Figures

Similar articles
-
Evolution of Telomeres in Schizosaccharomyces pombe and Its Possible Relationship to the Diversification of Telomere Binding Proteins.PLoS One. 2016 Apr 21;11(4):e0154225. doi: 10.1371/journal.pone.0154225. eCollection 2016. PLoS One. 2016. PMID: 27101289 Free PMC article.
-
Arabidopsis thaliana telomeric DNA-binding protein 1 is required for telomere length homeostasis and its Myb-extension domain stabilizes plant telomeric DNA binding.Nucleic Acids Res. 2007;35(4):1333-42. doi: 10.1093/nar/gkm043. Epub 2007 Jan 31. Nucleic Acids Res. 2007. PMID: 17272298 Free PMC article.
-
Telomere and telomerase biology.Prog Mol Biol Transl Sci. 2014;125:1-40. doi: 10.1016/B978-0-12-397898-1.00001-3. Prog Mol Biol Transl Sci. 2014. PMID: 24993696 Review.
-
The levels of telomere-binding proteins in human tumours and therapeutic implications.Eur J Cancer. 2009 Mar;45(4):536-50. doi: 10.1016/j.ejca.2008.11.014. Epub 2008 Dec 27. Eur J Cancer. 2009. PMID: 19114299 Review.
-
Evolution of Arabidopsis protection of telomeres 1 alters nucleic acid recognition and telomerase regulation.Nucleic Acids Res. 2016 Nov 16;44(20):9821-9830. doi: 10.1093/nar/gkw807. Epub 2016 Sep 19. Nucleic Acids Res. 2016. PMID: 27651456 Free PMC article.
Cited by
-
Evolution of Telomeres in Schizosaccharomyces pombe and Its Possible Relationship to the Diversification of Telomere Binding Proteins.PLoS One. 2016 Apr 21;11(4):e0154225. doi: 10.1371/journal.pone.0154225. eCollection 2016. PLoS One. 2016. PMID: 27101289 Free PMC article.
-
Telomeric Repeat-Binding Factor Homologs in Entamoeba histolytica: New Clues for Telomeric Research.Front Cell Infect Microbiol. 2018 Oct 2;8:341. doi: 10.3389/fcimb.2018.00341. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 30333961 Free PMC article.
-
Tieing together loose ends: telomere instability in cancer and aging.Mol Oncol. 2022 Sep;16(18):3380-3396. doi: 10.1002/1878-0261.13299. Epub 2022 Aug 16. Mol Oncol. 2022. PMID: 35920280 Free PMC article. Review.
-
Trypanosoma brucei UMSBP2 is a single-stranded telomeric DNA binding protein essential for chromosome end protection.Nucleic Acids Res. 2018 Sep 6;46(15):7757-7771. doi: 10.1093/nar/gky597. Nucleic Acids Res. 2018. PMID: 30007364 Free PMC article.
-
Double-stranded telomeric DNA binding proteins: Diversity matters.Cell Cycle. 2017;16(17):1568-1577. doi: 10.1080/15384101.2017.1356511. Epub 2017 Jul 27. Cell Cycle. 2017. PMID: 28749196 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

