Cellular Senescence as the Causal Nexus of Aging
- PMID: 26904101
- PMCID: PMC4751276
- DOI: 10.3389/fgene.2016.00013
Cellular Senescence as the Causal Nexus of Aging
Abstract
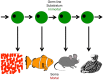
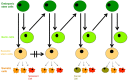
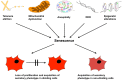
In this paper we present cellular senescence as the ultimate driver of the aging process, as a "causal nexus" that bridges microscopic subcellular damage with the phenotypic, macroscopic effect of aging. It is important to understand how the various types of subcellular damage correlated with the aging process lead to the larger, visible effects of anatomical aging. While it has always been assumed that subcellular damage (cause) results in macroscopic aging (effect), the bridging link between the two has been hard to define. Here, we propose that this bridge, which we term the "causal nexus", is in fact cellular senescence. The subcellular damage itself does not directly cause the visible signs of aging, but rather, as the damage accumulates and reaches a critical mass, cells cease to proliferate and acquire the deleterious "senescence-associated secretory phenotype" (SASP) which then leads to the macroscopic consequences of tissue breakdown to create the physiologically aged phenotype. Thus senescence is a precondition for anatomical aging, and this explains why aging is a gradual process that remains largely invisible during most of its progression. The subcellular damage includes shortening of telomeres, damage to mitochondria, aneuploidy, and DNA double-strand breaks triggered by various genetic, epigenetic, and environmental factors. Damage pathways acting in isolation or in concert converge at the causal nexus of cellular senescence. In each species some types of damage can be more causative than in others and operate at a variable pace; for example, telomere erosion appears to be a primary cause in human cells, whereas activation of tumor suppressor genes is more causative in rodents. Such species-specific mechanisms indicate that despite different initial causes, most of aging is traced to a single convergent causal nexus: senescence. The exception is in some invertebrate species that escape senescence, and in non-dividing cells such as neurons, where senescence still occurs, but results in the SASP rather than loss of proliferation plus SASP. Aging currently remains an inevitable endpoint for most biological organisms, but the field of cellular senescence is primed for a renaissance and as our understanding of aging is refined, strategies capable of decelerating the aging process will emerge.
Keywords: aging; august weismann; causal nexus; germline; immortality; mortality; proliferation; senescence.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

