Myeloablative Conditioning with PBSC Grafts for T Cell-Replete Haploidentical Donor Transplantation Using Posttransplant Cyclophosphamide
- PMID: 26904123
- PMCID: PMC4745340
- DOI: 10.1155/2016/9736564
Myeloablative Conditioning with PBSC Grafts for T Cell-Replete Haploidentical Donor Transplantation Using Posttransplant Cyclophosphamide
Abstract
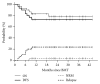
Relapse is the main cause of treatment failure after nonmyeloablative haploidentical transplant (haplo-HSCT). In an attempt to reduce relapse, we have developed a myeloablative (MA) haplo-HSCT approach utilizing posttransplant cyclophosphamide (PT/Cy) and peripheral blood stem cells as the stem cell source. We summarize the results of two consecutive clinical trials, using a busulfan-based (n = 20) and a TBI-based MA preparative regimen (n = 30), and analyze a larger cohort of 64 patients receiving MA haplo-HSCT. All patients have engrafted with full donor chimerism and no late graft failures. Grade III-IV acute GVHD and moderate-severe chronic GVHD occurred in 23% and 30%, respectively. One-year NRM was 10%. Predicted three-year overall survival, disease-free survival, and relapse were 53%, 53%, and 26%, respectively, in all patients and 79%, 74%, and 9%, respectively, in patients with a low/intermediate disease risk index (DRI). In multivariate analysis, DRI was the most significant predictor of survival and relapse. Use of TBI (versus busulfan) had no significant impact on survival but was associated with significantly less BK virus-associated hemorrhagic cystitis. We contrast our results with other published reports of MA haplo-HSCT PT/Cy in the literature and attempt to define the comparative utility of MA haplo-HSCT to other methods of transplantation.
Figures
References
-
- Szydlo R., Goldman J. M., Klein J. P., et al. Results of allogeneic bone marrow transplants for leukemia using donors other than HLA-identical siblings. Journal of Clinical Oncology. 1997;15(5):1767–1777. - PubMed
-
- Luznik L., O'Donnell P. V., Symons H. J., et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biology of Blood and Marrow Transplantation. 2008;14(6):641–650. doi: 10.1016/j.bbmt.2008.03.005. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources