Chemokine (C-X-C) Ligand 12 Facilitates Trafficking of Donor Spermatogonial Stem Cells
- PMID: 26904129
- PMCID: PMC4745625
- DOI: 10.1155/2016/5796305
Chemokine (C-X-C) Ligand 12 Facilitates Trafficking of Donor Spermatogonial Stem Cells
Abstract
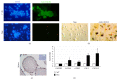
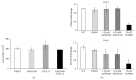
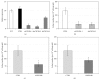
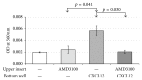
The chemokine (C-X-C) receptor type 4 (CXCR4) is an early marker of primordial germ cells (PGCs) essential for their migration and colonization of the gonads. In spermatogonial stem cells (SSCs), the expression of CXCR4 is promoted by the self-renewal factor, glial cell line-derived neurotrophic factor (GDNF). Here, we demonstrate an important role of CXCR4 during donor mouse SSCs reoccupation of the endogenous niche in recipient testis. Silencing of CXCR4 expression in mouse SSCs dramatically reduced the number of donor stem cell-derived colonies, whereas colony morphology and spermatogenesis were comparable to controls. Inhibition of CXCR4 signaling using a small molecule inhibitor (AMD3100) during the critical window of homing also significantly lowered the efficiency of donor-derived SSCs to establish spermatogenic colonies in recipient mice; however, the self-renewal of SSCs was not affected by exposure to AMD3100. Rather, in vitro migration assays demonstrate the influence of CXCR4-CXCL12 signaling in promoting germ cell migration. Together, these studies suggest that CXCR4-CXCL12 signaling functions to promote homing of SSCs towards the stem cell niche and plays a critical role in reestablishing spermatogenesis.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

