Effects of Charged Particles on Human Tumor Cells
- PMID: 26904502
- PMCID: PMC4751258
- DOI: 10.3389/fonc.2016.00023
Effects of Charged Particles on Human Tumor Cells
Abstract
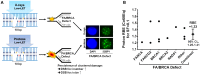
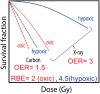
The use of charged particle therapy in cancer treatment is growing rapidly, in large part because the exquisite dose localization of charged particles allows for higher radiation doses to be given to tumor tissue while normal tissues are exposed to lower doses and decreased volumes of normal tissues are irradiated. In addition, charged particles heavier than protons have substantial potential clinical advantages because of their additional biological effects, including greater cell killing effectiveness, decreased radiation resistance of hypoxic cells in tumors, and reduced cell cycle dependence of radiation response. These biological advantages depend on many factors, such as endpoint, cell or tissue type, dose, dose rate or fractionation, charged particle type and energy, and oxygen concentration. This review summarizes the unique biological advantages of charged particle therapy and highlights recent research and areas of particular research needs, such as quantification of relative biological effectiveness (RBE) for various tumor types and radiation qualities, role of genetic background of tumor cells in determining response to charged particles, sensitivity of cancer stem-like cells to charged particles, role of charged particles in tumors with hypoxic fractions, and importance of fractionation, including use of hypofractionation, with charged particles.
Keywords: altered fractionation; cancer stem cells; carbon-ion therapy; charged particles; clustered DNA damage; hypoxic radioresistance; proton therapy; relative biological effectiveness.
Figures







References
-
- Skarsgard LD. Radiobiology with heavy charged particles: a historical review. Phys Med (1998) 14(Suppl. 1):1–19. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

