The Many Faces of IpaB
- PMID: 26904511
- PMCID: PMC4746235
- DOI: 10.3389/fcimb.2016.00012
The Many Faces of IpaB
Abstract
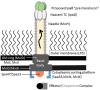
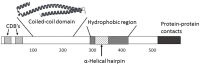
The type III secretion system (T3SS) is Shigella's most important virulence factor. The T3SS apparatus (T3SA) is comprised of an envelope-spanning basal body and an external needle topped by a tip complex protein called IpaD. This nanomachine is used to deliver effector proteins into host cells to promote pathogen entry. A key component of the matured T3SS needle tip complex is the translocator protein IpaB. IpaB can exist in multiple states when prepared as a recombinant protein, however, it has also been described as having additional roles in Shigella pathogenesis. This mini-review will briefly describe some of the features of IpaB as a T3SS needle tip protein, as a pore-forming translocator protein and as an effector protein. Reflection on the potential importance of the different in vitro states of IpaB on its function and importance in serotype-independent vaccines is also provided.
Keywords: IpaB; Shigella; invasion plasmid antigen; pathogenesis; type III secretion.
Figures
Similar articles
-
Molecular and Cellular Mechanisms of Shigella flexneri Dissemination.Front Cell Infect Microbiol. 2016 Mar 11;6:29. doi: 10.3389/fcimb.2016.00029. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 27014639 Free PMC article. Review.
-
Topology and Contribution to the Pore Channel Lining of Plasma Membrane-Embedded Shigella flexneri Type 3 Secretion Translocase IpaB.mBio. 2021 Dec 21;12(6):e0302121. doi: 10.1128/mBio.03021-21. Epub 2021 Nov 23. mBio. 2021. PMID: 34809452 Free PMC article.
-
Using disruptive insertional mutagenesis to identify the in situ structure-function landscape of the Shigella translocator protein IpaB.Protein Sci. 2018 Aug;27(8):1392-1406. doi: 10.1002/pro.3428. Epub 2018 May 3. Protein Sci. 2018. PMID: 29672980 Free PMC article.
-
N-terminus of IpaB provides a potential anchor to the Shigella type III secretion system tip complex protein IpaD.Biochemistry. 2013 Dec 10;52(49):8790-9. doi: 10.1021/bi400755f. Epub 2013 Nov 20. Biochemistry. 2013. PMID: 24236510 Free PMC article.
-
Implications of Spatiotemporal Regulation of Shigella flexneri Type Three Secretion Activity on Effector Functions: Think Globally, Act Locally.Front Cell Infect Microbiol. 2016 Mar 9;6:28. doi: 10.3389/fcimb.2016.00028. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 27014638 Free PMC article. Review.
Cited by
-
The T3SS of Shigella: Expression, Structure, Function, and Role in Vacuole Escape.Microorganisms. 2020 Dec 5;8(12):1933. doi: 10.3390/microorganisms8121933. Microorganisms. 2020. PMID: 33291504 Free PMC article. Review.
-
Interfacial amino acids support Spa47 oligomerization and shigella type three secretion system activation.Proteins. 2019 Nov;87(11):931-942. doi: 10.1002/prot.25754. Epub 2019 Jun 21. Proteins. 2019. PMID: 31162724 Free PMC article.
-
Structural Insights of Shigella Translocator IpaB and Its Chaperone IpgC in Solution.Front Cell Infect Microbiol. 2021 Apr 29;11:673122. doi: 10.3389/fcimb.2021.673122. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33996640 Free PMC article.
-
An Experimental Adult Zebrafish Model for Shigella Pathogenesis, Transmission, and Vaccine Efficacy Studies.Microbiol Spectr. 2022 Jun 29;10(3):e0034722. doi: 10.1128/spectrum.00347-22. Epub 2022 May 23. Microbiol Spectr. 2022. PMID: 35604149 Free PMC article.
-
Efficient production of immunologically active Shigella invasion plasmid antigens IpaB and IpaH using a cell-free expression system.Appl Microbiol Biotechnol. 2022 Jan;106(1):401-414. doi: 10.1007/s00253-021-11701-4. Epub 2021 Dec 21. Appl Microbiol Biotechnol. 2022. PMID: 34932164 Free PMC article.
References
-
- Adam P. R., Dickenson N. E., Greenwood J. C., II, Picking W. L., Picking W. D. (2014). Influence of oligomerization state on the structural properties of invasion plasmid antigen B from Shigella flexneri in the presence and absence of phospholipid membranes. Proteins 82, 3013–3022. 10.1002/prot.24662 - DOI - PMC - PubMed
-
- Adam P. R., Picking W. D. (2015). Shigella and shigellosis in Shigella: Molecular and Cellular Biology, eds Picking W. D., Picking W. L. (Poole: Caister Academic Press; ), 7–26.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

