Brucella suis Vaccine Strain 2 Induces Endoplasmic Reticulum Stress that Affects Intracellular Replication in Goat Trophoblast Cells In vitro
- PMID: 26904517
- PMCID: PMC4746994
- DOI: 10.3389/fcimb.2016.00019
Brucella suis Vaccine Strain 2 Induces Endoplasmic Reticulum Stress that Affects Intracellular Replication in Goat Trophoblast Cells In vitro
Abstract
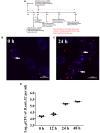
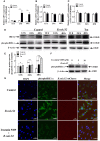
Brucella has been reported to impair placental trophoblasts, a cellular target where Brucella efficiently replicates in association with the endoplasmic reticulum (ER), and ultimately trigger abortion in pregnant animals. However, the precise effects of Brucella on trophoblast cells remain unclear. Here, we describe the infection and replication of Brucella suis vaccine strain 2 (B.suis.S2) in goat trophoblast cells (GTCs) and the cellular and molecular responses induced in vitro. Our studies demonstrated that B.suis.S2 was able to infect and proliferate to high titers, hamper the proliferation of GTCs and induce apoptosis due to ER stress. Tunicamycin (Tm), a pharmacological chaperone that strongly mounts ER stress-induced apoptosis, inhibited B.suis.S2 replication in GTCs. In addition, 4 phenyl butyric acid (4-PBA), a pharmacological chaperone that alleviates ER stress-induced apoptosis, significantly enhanced B.suis.S2 replication in GTCs. The Unfolded Protein Response (UPR) chaperone molecule GRP78 also promoted B.suis.S2 proliferation in GTCs by inhibiting ER stress-induced apoptosis. We also discovered that the IRE1 pathway, but not the PERK or ATF6 pathway, was activated in the process. However, decreasing the expression of phosphoIRE1α and IRE1α proteins with Irestatin 9389 (IRE1 antagonist) in GTCs did not affect the proliferation of B.suis.S2. Although GTC implantation was not affected upon B.suis.S2 infection, progesterone secretion was suppressed, and prolactin and estrogen secretion increased; these effects were accompanied by changes in the expression of genes encoding key steroidogenic enzymes. This study systematically explored the mechanisms of abortion in Brucella infection from the viewpoint of pathogen invasion, ER stress and reproductive endocrinology. Our findings may provide new insight for understanding the mechanisms involved in goat abortions caused by Brucella infection.
Keywords: B.suis.S2; apoptosis; endoplasmic reticulum stress; goat trophoblast cells; infection.
Figures
Similar articles
-
VceC Mediated IRE1 Pathway and Inhibited CHOP-induced Apoptosis to Support Brucella Replication in Goat Trophoblast Cells.Int J Mol Sci. 2019 Aug 22;20(17):4104. doi: 10.3390/ijms20174104. Int J Mol Sci. 2019. PMID: 31443507 Free PMC article.
-
Brucella suis vaccine strain S2-infected immortalized caprine endometrial epithelial cell lines induce non-apoptotic ER-stress.Cell Stress Chaperones. 2015 May;20(3):399-409. doi: 10.1007/s12192-014-0564-x. Epub 2015 Jan 30. Cell Stress Chaperones. 2015. PMID: 25633898 Free PMC article.
-
Brucella abortus Infection of Placental Trophoblasts Triggers Endoplasmic Reticulum Stress-Mediated Cell Death and Fetal Loss via Type IV Secretion System-Dependent Activation of CHOP.mBio. 2019 Jul 23;10(4):e01538-19. doi: 10.1128/mBio.01538-19. mBio. 2019. PMID: 31337727 Free PMC article.
-
The endoplasmic reticulum stress response: A link with tuberculosis?Tuberculosis (Edinb). 2016 Mar;97:52-6. doi: 10.1016/j.tube.2015.12.009. Epub 2016 Jan 2. Tuberculosis (Edinb). 2016. PMID: 26980496 Review.
-
Cell death induced by endoplasmic reticulum stress.FEBS J. 2016 Jul;283(14):2640-52. doi: 10.1111/febs.13598. Epub 2015 Dec 19. FEBS J. 2016. PMID: 26587781 Review.
Cited by
-
Brucella activates the host RIDD pathway to subvert BLOS1-directed immune defense.Elife. 2022 May 19;11:e73625. doi: 10.7554/eLife.73625. Elife. 2022. PMID: 35587649 Free PMC article.
-
Cell and Tissue Tropism of Brucella spp.Infect Immun. 2023 May 16;91(5):e0006223. doi: 10.1128/iai.00062-23. Epub 2023 Apr 27. Infect Immun. 2023. PMID: 37129522 Free PMC article. Review.
-
Estrogen inhibits endoplasmic reticulum stress and ameliorates myocardial ischemia/reperfusion injury in rats by upregulating SERCA2a.Cell Commun Signal. 2022 Mar 24;20(1):38. doi: 10.1186/s12964-022-00842-2. Cell Commun Signal. 2022. PMID: 35331264 Free PMC article.
-
A LysR Transcriptional Regulator Manipulates Macrophage Autophagy Flux During Brucella Infection.Front Cell Infect Microbiol. 2022 Mar 22;12:858173. doi: 10.3389/fcimb.2022.858173. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35392609 Free PMC article.
-
Comparative Review of Brucellosis in Small Domestic Ruminants.Front Vet Sci. 2022 May 12;9:887671. doi: 10.3389/fvets.2022.887671. eCollection 2022. Front Vet Sci. 2022. PMID: 35647101 Free PMC article. Review.
References
-
- Barquero-Calvo E., Mora-Cartín R., Arce-Gorvel V., de Diego J. L., Chacón-Díaz C., Chaves-Olarte E., et al. . (2015). Brucella abortus induces the premature death of human neutrophils through the action of its lipopolysaccharide. PLoS Pathog. 11:e1004853. 10.1371/journal.ppat.1004853 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

