Solvent-Free Lipase-Catalyzed Synthesis of Diacylgycerols as Low-Calorie Food Ingredients
- PMID: 26904539
- PMCID: PMC4748054
- DOI: 10.3389/fbioe.2016.00006
Solvent-Free Lipase-Catalyzed Synthesis of Diacylgycerols as Low-Calorie Food Ingredients
Abstract
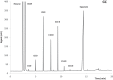
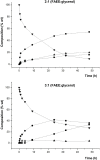
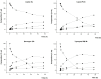
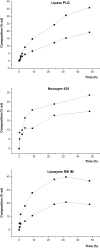
Problems derived from obesity and overweight have recently promoted the development of fat substitutes and other low-calorie foods. On the one hand, fats with short- and medium-chain fatty acids are a source of quick energy, easily hydrolyzable and hardly stored as fat. Furthermore, 1,3-diacylglycerols are not hydrolyzed to 2-monoacylglycerols in the gastrointestinal tract, reducing the formation of chylomicron and lowers the serum level of triacylglycerols by decreasing its resynthesis in the enterocyte. In this work, these two effects were combined to synthesize short- and medium-chain 1,3-diacylglycerols, leading to a product with great potential as for their low-calorie properties. Lipase-catalyzed transesterification reactions were performed between short- and medium-chain fatty acid ethyl esters and glycerol. Different variables were investigated, such as the type of biocatalyst, the molar ratio FAEE:glycerol, the adsorption of glycerol on silica gel, or the addition of lecithin. Best reaction conditions were evaluated considering the percentage of 1,3-DAG produced and the reaction rate. Except Novozym 435 (Candida antarctica), other lipases required the adsorption of glycerol on silica gel to form acylglycerols. Lipases that gave the best results with adsorption were Novozym 435 and Lipozyme RM IM (Rhizomucor miehei) with 52 and 60.7% DAG at 32 h, respectively. Because of its specificity for sn-1 and sn-3 positions, lipases leading to a higher proportion of 1,3-DAG vs. 1,2-DAG were Lipozyme RM IM (39.8 and 20.9%, respectively) and Lipase PLG (Alcaligenes sp.) (35.9 and 19.3%, respectively). By adding 1% (w/w) of lecithin to the reaction with Novozym 435 and raw glycerol, the reaction rate was considerably increased from 41.7 to 52.8% DAG at 24 h.
Keywords: diacylglycerol; fatty acid ethyl ester; lipase; medium-chain fatty acid; solvent-free; transesterification.
Figures


Similar articles
-
Solvent-free lipase-catalyzed preparation of diacylglycerols.J Agric Food Chem. 2004 Aug 25;52(17):5347-53. doi: 10.1021/jf0400819. J Agric Food Chem. 2004. PMID: 15315368
-
Steryl and stanyl esters of fatty acids by solvent-free esterification and transesterification in vacuo using lipases from Rhizomucor miehei, Candida antarctica, and Carica papaya.J Agric Food Chem. 2001 Nov;49(11):5210-6. doi: 10.1021/jf0107407. J Agric Food Chem. 2001. PMID: 11714305
-
Lipase-catalyzed incorporation of different Fatty acids into tripalmitin-enriched triacylglycerols: effect of reaction parameters.J Agric Food Chem. 2012 Mar 7;60(9):2377-84. doi: 10.1021/jf300088c. Epub 2012 Feb 23. J Agric Food Chem. 2012. PMID: 22360498
-
Preparation and characterization of diacylglycerol via ultrasound-assisted enzyme-catalyzed transesterification of lard with glycerol monolaurate.Ultrason Sonochem. 2023 May;95:106354. doi: 10.1016/j.ultsonch.2023.106354. Epub 2023 Mar 4. Ultrason Sonochem. 2023. PMID: 36898248 Free PMC article. Review.
-
Selective production of functional sn-1,3-diacylglycerol by microbial lipases: A comprehensive review.Food Chem. 2025 Jul 30;481:144017. doi: 10.1016/j.foodchem.2025.144017. Epub 2025 Mar 27. Food Chem. 2025. PMID: 40179503 Review.
Cited by
-
Enzymatic Synthesis of Diacylglycerol-Enriched Oil by Two-Step Vacuum-Mediated Conversion of Fatty Acid Ethyl Ester and Fatty Acid From Soy Sauce By-Product Oil as Lipid-Lowering Functional Oil.Front Nutr. 2022 Apr 27;9:884829. doi: 10.3389/fnut.2022.884829. eCollection 2022. Front Nutr. 2022. PMID: 35571905 Free PMC article.
-
Argan Oil as a Rich Source of Linoleic Fatty Acid for Dietetic Structured Lipids Production.Life (Basel). 2021 Oct 20;11(11):1114. doi: 10.3390/life11111114. Life (Basel). 2021. PMID: 34832990 Free PMC article.
-
A review on preparation and application of low-calorie structured lipids in food system.Food Sci Biotechnol. 2024 Aug 26;34(1):49-64. doi: 10.1007/s10068-024-01689-8. eCollection 2025 Jan. Food Sci Biotechnol. 2024. PMID: 39758727 Free PMC article. Review.
-
Genetically modified lipases as biocatalysts for diacylglycerol production in the food industry: a critical review.Arch Microbiol. 2025 Jun 4;207(7):166. doi: 10.1007/s00203-025-04361-9. Arch Microbiol. 2025. PMID: 40464989 Review.
-
Future of Structured Lipids: Enzymatic Synthesis and Their New Applications in Food Systems.Foods. 2022 Aug 10;11(16):2400. doi: 10.3390/foods11162400. Foods. 2022. PMID: 36010399 Free PMC article. Review.
References
-
- Akoh C., Yee L. (1997). Enzymatic synthesis of position-specific low-calorie structured lipids. J. Am. Oil Chem. Soc. 74, 1409–1413.10.1007/s11746-997-0245-3 - DOI
-
- Akoh C. C. (1998). Fat replacers. Food Technol. 52, 47–53.
-
- Bach A. C., Babayan V. K. (1982). Medium-chain triglycerides: an update. Am. J. Clin. Nutr. 36, 950–962. - PubMed
-
- Berger M., Laumen K., Schneider M. (1992a). Enzymatic esterification of glycerol I. Lipase-catalyzed synthesis of regioisomerically pure 1,3-sn-diacylglycerols. J. Am. Oil Chem. Soc. 69, 955–960.10.1007/BF02541058 - DOI
-
- Berger M., Laumen K., Schneider M. (1992b). Lipase-catalyzed esterification of hydrophilic diols in organic solvents. Biotechnol. Lett. 14, 553–558.10.1007/BF01023939 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

