Solvent-Free Lipase-Catalyzed Synthesis of Diacylgycerols as Low-Calorie Food Ingredients
- PMID: 26904539
- PMCID: PMC4748054
- DOI: 10.3389/fbioe.2016.00006
Solvent-Free Lipase-Catalyzed Synthesis of Diacylgycerols as Low-Calorie Food Ingredients
Abstract
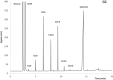
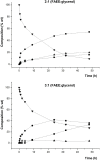
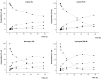
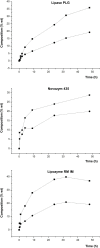
Problems derived from obesity and overweight have recently promoted the development of fat substitutes and other low-calorie foods. On the one hand, fats with short- and medium-chain fatty acids are a source of quick energy, easily hydrolyzable and hardly stored as fat. Furthermore, 1,3-diacylglycerols are not hydrolyzed to 2-monoacylglycerols in the gastrointestinal tract, reducing the formation of chylomicron and lowers the serum level of triacylglycerols by decreasing its resynthesis in the enterocyte. In this work, these two effects were combined to synthesize short- and medium-chain 1,3-diacylglycerols, leading to a product with great potential as for their low-calorie properties. Lipase-catalyzed transesterification reactions were performed between short- and medium-chain fatty acid ethyl esters and glycerol. Different variables were investigated, such as the type of biocatalyst, the molar ratio FAEE:glycerol, the adsorption of glycerol on silica gel, or the addition of lecithin. Best reaction conditions were evaluated considering the percentage of 1,3-DAG produced and the reaction rate. Except Novozym 435 (Candida antarctica), other lipases required the adsorption of glycerol on silica gel to form acylglycerols. Lipases that gave the best results with adsorption were Novozym 435 and Lipozyme RM IM (Rhizomucor miehei) with 52 and 60.7% DAG at 32 h, respectively. Because of its specificity for sn-1 and sn-3 positions, lipases leading to a higher proportion of 1,3-DAG vs. 1,2-DAG were Lipozyme RM IM (39.8 and 20.9%, respectively) and Lipase PLG (Alcaligenes sp.) (35.9 and 19.3%, respectively). By adding 1% (w/w) of lecithin to the reaction with Novozym 435 and raw glycerol, the reaction rate was considerably increased from 41.7 to 52.8% DAG at 24 h.
Keywords: diacylglycerol; fatty acid ethyl ester; lipase; medium-chain fatty acid; solvent-free; transesterification.
Figures


References
-
- Akoh C., Yee L. (1997). Enzymatic synthesis of position-specific low-calorie structured lipids. J. Am. Oil Chem. Soc. 74, 1409–1413. 10.1007/s11746-997-0245-3 - DOI
-
- Akoh C. C. (1998). Fat replacers. Food Technol. 52, 47–53.
-
- Bach A. C., Babayan V. K. (1982). Medium-chain triglycerides: an update. Am. J. Clin. Nutr. 36, 950–962. - PubMed
-
- Berger M., Laumen K., Schneider M. (1992a). Enzymatic esterification of glycerol I. Lipase-catalyzed synthesis of regioisomerically pure 1,3-sn-diacylglycerols. J. Am. Oil Chem. Soc. 69, 955–960. 10.1007/BF02541058 - DOI
-
- Berger M., Laumen K., Schneider M. (1992b). Lipase-catalyzed esterification of hydrophilic diols in organic solvents. Biotechnol. Lett. 14, 553–558. 10.1007/BF01023939 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

