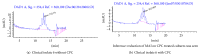The Assessment of Viability of M. Tuberculosis after Exposure to CPC Using Different Methods
- PMID: 26904736
- PMCID: PMC4745468
- DOI: 10.1155/2014/564109
The Assessment of Viability of M. Tuberculosis after Exposure to CPC Using Different Methods
Abstract
Settings. National Institute for Research in Tuberculosis, Chennai. Objective. To assess the proportion of metabolically active cells of Mycobacterium tuberculosis after exposed to CPC using FDA-EB vital staining and viable counts on LJ medium. Mycolic acid content in M. tuberculosis after exposure to CPC was estimated using HPLC. Methods. Clinical isolates of M. tuberculosis and standard reference strain M. tuberculosis H37Rv were used for FDA-EB, viable count, and HPLC. Results. FDA/EB consistently stained 70-90% of log phase cells as green and the remaining cells as red-orange. After CPC treatment, 65-70% of the cells stained red-orange. The viability counts were comparable to 0-day controls. Synthesis of mycolic acids in mycobacteria was reduced when exposed to CPC using HPLC due to the decreased metabolic activity of the organisms. Conclusion. The cells are metabolically inactive during storage with CPC but these cells grew well on LJ medium after removal of CPC. The viability of M. tuberculosis was maintained in CPC with minimal reduction. Mycolic acid content was reduced if the cells of M. tuberculosis were treated with CPC for 7 days. All the above findings provide yet another evidence for the damage of cell wall of M. tuberculosis.
Figures
Similar articles
-
Performance of OMNIgene•SPUTUM (DNA Genotek) and cetylpyridinium chloride for sputum storage prior to mycobacterial culture.J Med Microbiol. 2018 Jun;67(6):798-805. doi: 10.1099/jmm.0.000745. J Med Microbiol. 2018. PMID: 29717969
-
[Standardization of laboratory tests for tuberculosis and their proficiency testing].Kekkaku. 2003 Aug;78(8):541-51. Kekkaku. 2003. PMID: 14509226 Review. Japanese.
-
Antimycobacterial activities of isoxyl and new derivatives through the inhibition of mycolic acid synthesis.Antimicrob Agents Chemother. 1999 May;43(5):1042-51. doi: 10.1128/AAC.43.5.1042. Antimicrob Agents Chemother. 1999. PMID: 10223912 Free PMC article.
-
In vitro activity of a novel antimycobacterial compound, N-octanesulfonylacetamide, and its effects on lipid and mycolic acid synthesis.Antimicrob Agents Chemother. 2001 Apr;45(4):1143-50. doi: 10.1128/AAC.45.4.1143-1150.2001. Antimicrob Agents Chemother. 2001. PMID: 11257028 Free PMC article.
-
Towards understanding the functional diversity of cell wall mycolic acids of Mycobacterium tuberculosis.Prog Lipid Res. 2012 Oct;51(4):325-39. doi: 10.1016/j.plipres.2012.05.002. Epub 2012 May 30. Prog Lipid Res. 2012. PMID: 22659327 Review.
Cited by
-
Evaluation of six decontamination procedures for isolation of Mycobacterium avium complex from avian feces.PLoS One. 2018 Aug 10;13(8):e0202034. doi: 10.1371/journal.pone.0202034. eCollection 2018. PLoS One. 2018. PMID: 30096205 Free PMC article.
References
-
- World Health Organization. Global Tuberculosis Control: Surveillance, Planning and Financing. Geneva, Switzerland: WHO; 2006.
-
- World Health Organization, Stop TB Partnership. The Stop TB Strategy: Building on and enhancing DOTS to meet the TB-related Millennium Development Goals. Geneva, Switzerland: WHO; 2006.
-
- World Health Organization. The Global Plan to Stop TB, 2006–2015. Actions for life-towards a world free of tuberculosis. Geneva, Switzerland: WHO; 2006. - PubMed
-
- Islam S., Rahman F., Munshi S. K., Ahmed J., Kamal S. M. M., Noor R. Use of fluorescein diacetate (FDA) staining to detect viable Mycobacterium tuberculosis . Bangladesh Journal of Medical Science. 2012;11(4)
-
- Harada S., Numata N. Application of FDA/EB staining for the detection of viable or non-viable mycobacteria in clinical specimens. Kekkaku. 1992;67(2):113–117. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources