Real-Time Control of a Neuroprosthetic Hand by Magnetoencephalographic Signals from Paralysed Patients
- PMID: 26904967
- PMCID: PMC4764841
- DOI: 10.1038/srep21781
Real-Time Control of a Neuroprosthetic Hand by Magnetoencephalographic Signals from Paralysed Patients
Erratum in
-
Corrigendum: Real-Time Control of a Neuroprosthetic Hand by Magnetoencephalographic Signals from Paralysed Patients.Sci Rep. 2016 Oct 19;6:34970. doi: 10.1038/srep34970. Sci Rep. 2016. PMID: 27759013 Free PMC article. No abstract available.
Abstract
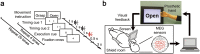
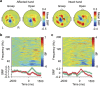
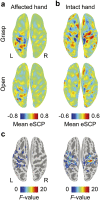
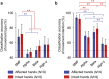
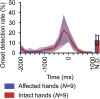
Neuroprosthetic arms might potentially restore motor functions for severely paralysed patients. Invasive measurements of cortical currents using electrocorticography have been widely used for neuroprosthetic control. Moreover, magnetoencephalography (MEG) exhibits characteristic brain signals similar to those of invasively measured signals. However, it remains unclear whether non-invasively measured signals convey enough motor information to control a neuroprosthetic hand, especially for severely paralysed patients whose sensorimotor cortex might be reorganized. We tested an MEG-based neuroprosthetic system to evaluate the accuracy of using cortical currents in the sensorimotor cortex of severely paralysed patients to control a prosthetic hand. The patients attempted to grasp with or open their paralysed hand while the slow components of MEG signals (slow movement fields; SMFs) were recorded. Even without actual movements, the SMFs of all patients indicated characteristic spatiotemporal patterns similar to actual movements, and the SMFs were successfully used to control a neuroprosthetic hand in a closed-loop condition. These results demonstrate that the slow components of MEG signals carry sufficient information to classify movement types. Successful control by paralysed patients suggests the feasibility of using an MEG-based neuroprosthetic hand to predict a patient's ability to control an invasive neuroprosthesis via the same signal sources as the non-invasive method.
Figures
Similar articles
-
Closed-Loop Control of a Neuroprosthetic Hand by Magnetoencephalographic Signals.PLoS One. 2015 Jul 2;10(7):e0131547. doi: 10.1371/journal.pone.0131547. eCollection 2015. PLoS One. 2015. PMID: 26134845 Free PMC article.
-
Real-time control of a prosthetic hand using human electrocorticography signals.J Neurosurg. 2011 Jun;114(6):1715-22. doi: 10.3171/2011.1.JNS101421. Epub 2011 Feb 11. J Neurosurg. 2011. PMID: 21314273
-
Restoring cortical control of functional movement in a human with quadriplegia.Nature. 2016 May 12;533(7602):247-50. doi: 10.1038/nature17435. Epub 2016 Apr 13. Nature. 2016. PMID: 27074513
-
Brain-computer interfaces: communication and restoration of movement in paralysis.J Physiol. 2007 Mar 15;579(Pt 3):621-36. doi: 10.1113/jphysiol.2006.125633. Epub 2007 Jan 18. J Physiol. 2007. PMID: 17234696 Free PMC article. Review.
-
Integrated technology for evaluation of brain function and neural plasticity.Phys Med Rehabil Clin N Am. 2004 Feb;15(1):263-306. doi: 10.1016/s1047-9651(03)00124-4. Phys Med Rehabil Clin N Am. 2004. PMID: 15029909 Review.
Cited by
-
Closed-Loop Hybrid Gaze Brain-Machine Interface Based Robotic Arm Control with Augmented Reality Feedback.Front Neurorobot. 2017 Oct 31;11:60. doi: 10.3389/fnbot.2017.00060. eCollection 2017. Front Neurorobot. 2017. PMID: 29163123 Free PMC article.
-
BCI training to move a virtual hand reduces phantom limb pain: A randomized crossover trial.Neurology. 2020 Jul 28;95(4):e417-e426. doi: 10.1212/WNL.0000000000009858. Epub 2020 Jul 16. Neurology. 2020. PMID: 32675074 Free PMC article. Clinical Trial.
-
Assistive Technologies for Individuals with a Disability from a Neurological Condition: A Narrative Review on the Multimodal Integration.Healthcare (Basel). 2025 Jul 1;13(13):1580. doi: 10.3390/healthcare13131580. Healthcare (Basel). 2025. PMID: 40648603 Free PMC article. Review.
-
Progress in Brain Computer Interface: Challenges and Opportunities.Front Syst Neurosci. 2021 Feb 25;15:578875. doi: 10.3389/fnsys.2021.578875. eCollection 2021. Front Syst Neurosci. 2021. PMID: 33716680 Free PMC article. Review.
-
Attempted Arm and Hand Movements can be Decoded from Low-Frequency EEG from Persons with Spinal Cord Injury.Sci Rep. 2019 May 9;9(1):7134. doi: 10.1038/s41598-019-43594-9. Sci Rep. 2019. PMID: 31073142 Free PMC article.
References
-
- Shoham S., Halgren E., Maynard E. M. & Normann R. A. Motor-cortical activity in tetraplegics. Nature 413, 793 (2001). - PubMed
-
- Ball T., Schulze-Bonhage A., Aertsen A. & Mehring C. Differential representation of arm movement direction in relation to cortical anatomy and function. J. Neural Eng. 6, 016006 (2009). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

