Cucurbitacin B inhibits the stemness and metastatic abilities of NSCLC via downregulation of canonical Wnt/β-catenin signaling axis
- PMID: 26905250
- PMCID: PMC4764833
- DOI: 10.1038/srep21860
Cucurbitacin B inhibits the stemness and metastatic abilities of NSCLC via downregulation of canonical Wnt/β-catenin signaling axis
Abstract
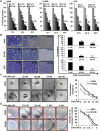
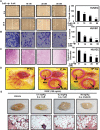
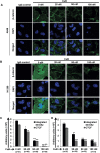
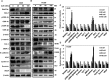
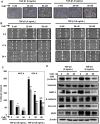
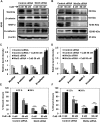
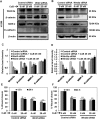
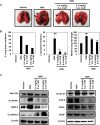
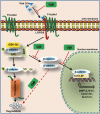
Lack of effective anti-metastatic drugs creates a major hurdle for metastatic lung cancer therapy. For successful lung cancer treatment, there is a strong need of newer therapeutics with metastasis-inhibitory potential. In the present study, we determined the anti-metastatic and anti-angiogenic potential of a natural plant triterpenoid, Cucurbitacin B (CuB) against non-small cell lung cancer (NSCLC) both in vitro and in vivo. CuB demonstrated a strong anti-migratory and anti-invasive ability against metastatic NSCLC at nanomolar concentrations. CuB also showed significant tumor angiogenesis-inhibitory effects as evidenced by the inhibition of migratory, invasive and tube-forming capacities of human umbilical vein endothelial cells. CuB-mediated inhibition of angiogenesis was validated by the inhibition of pre-existing vasculature in chick embryo chorio-allantoic membrane and matrigel plugs. Similarly, CuB inhibited the migratory behavior of TGF-β1-induced experimental EMT model. The CuB-mediated inhibition of metastasis and angiogenesis was attributable to the downregulation of Wnt/β-catenin signaling axis, validated by siRNA-knockdown of Wnt3 and Wnt3a. The CuB-mediated downregulation of Wnt/β-catenin signaling was also validated using 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK)-induced lung tumorigenesis model in vivo. Collectively, our findings suggest that CuB inhibited the metastatic abilities of NSCLC through the inhibition of Wnt/β-catenin signaling axis.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

