Trehalose inhibits solute carrier 2A (SLC2A) proteins to induce autophagy and prevent hepatic steatosis
- PMID: 26905426
- PMCID: PMC4816640
- DOI: 10.1126/scisignal.aac5472
Trehalose inhibits solute carrier 2A (SLC2A) proteins to induce autophagy and prevent hepatic steatosis
Abstract
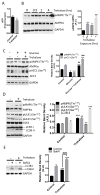
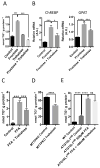
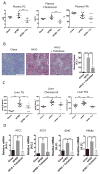
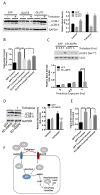
Trehalose is a naturally occurring disaccharide that has gained attention for its ability to induce cellular autophagy and mitigate diseases related to pathological protein aggregation. Despite decades of ubiquitous use as a nutraceutical, preservative, and humectant, its mechanism of action remains elusive. We showed that trehalose inhibited members of the SLC2A (also known as GLUT) family of glucose transporters. Trehalose-mediated inhibition of glucose transport induced AMPK (adenosine 5'-monophosphate-activated protein kinase)-dependent autophagy and regression of hepatic steatosis in vivo and a reduction in the accumulation of lipid droplets in primary murine hepatocyte cultures. Our data indicated that trehalose triggers beneficial cellular autophagy by inhibiting glucose transport.
Copyright © 2016, American Association for the Advancement of Science.
Conflict of interest statement
Competing interests: KHM is on the Scientific Board of Advisors for OvaScience.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- K12HD076224/HD/NICHD NIH HHS/United States
- R01-DK078187/DK/NIDDK NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- P30DK056341/DK/NIDDK NIH HHS/United States
- P41 GM103422/GM/NIGMS NIH HHS/United States
- R01 DK078187/DK/NIDDK NIH HHS/United States
- R01 HL038180/HL/NHLBI NIH HHS/United States
- K12HD000850-29/HD/NICHD NIH HHS/United States
- K12 HD000850/HD/NICHD NIH HHS/United States
- HL-38180/HL/NHLBI NIH HHS/United States
- P30DK52574/DK/NIDDK NIH HHS/United States
- DK-56260/DK/NIDDK NIH HHS/United States
- K12 HD076224/HD/NICHD NIH HHS/United States
- P60 DK020579/DK/NIDDK NIH HHS/United States
- R37 HL038180/HL/NHLBI NIH HHS/United States
- P30 DK020579/DK/NIDDK NIH HHS/United States
- R01 DK056260/DK/NIDDK NIH HHS/United States
- T32 GM007067/GM/NIGMS NIH HHS/United States
- P30 DK052574/DK/NIDDK NIH HHS/United States
- T32GM007067/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

