The role of kinetic context in apparent biased agonism at GPCRs
- PMID: 26905976
- PMCID: PMC4770093
- DOI: 10.1038/ncomms10842
The role of kinetic context in apparent biased agonism at GPCRs
Abstract
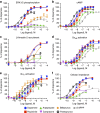
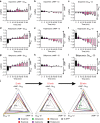
Biased agonism describes the ability of ligands to stabilize different conformations of a GPCR linked to distinct functional outcomes and offers the prospect of designing pathway-specific drugs that avoid on-target side effects. This mechanism is usually inferred from pharmacological data with the assumption that the confounding influences of observational (that is, assay dependent) and system (that is, cell background dependent) bias are excluded by experimental design and analysis. Here we reveal that 'kinetic context', as determined by ligand-binding kinetics and the temporal pattern of receptor-signalling processes, can have a profound influence on the apparent bias of a series of agonists for the dopamine D2 receptor and can even lead to reversals in the direction of bias. We propose that kinetic context must be acknowledged in the design and interpretation of studies of biased agonism.
Figures






References
-
- Kenakin T. & Christopoulos A. Signalling bias in new drug discovery: detection, quantification and therapeutic impact. Nat. Rev. Drug Discov. 12, 205–216 (2013). - PubMed
-
- Urban J. D. et al. Functional selectivity and classical concepts of quantitative pharmacology. J. Pharmacol. Exp. Ther. 320, 1–13 (2007). - PubMed
-
- Overington J. P., Al-Lazikani B. & Hopkins A. L. How many drug targets are there? Nat. Rev. Drug Discov. 5, 993–996 (2006). - PubMed
-
- Violin J. D., Crombie A. L., Soergel D. G. & Lark M. W. Erratum: biased ligands at G protein-coupled receptors: promise and progress. Trends Pharmacol. Sci. 35, 308–316 (2014). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

