Interleukin-1 Blockade in Acute Decompensated Heart Failure: A Randomized, Double-Blinded, Placebo-Controlled Pilot Study
- PMID: 26906034
- PMCID: PMC5749643
- DOI: 10.1097/FJC.0000000000000378
Interleukin-1 Blockade in Acute Decompensated Heart Failure: A Randomized, Double-Blinded, Placebo-Controlled Pilot Study
Abstract
Background: Heart failure is an inflammatory disease. Patients with acute decompensated heart failure (ADHF) exhibit significant inflammatory activity on admission. We hypothesized that Interleukin-1 blockade, with anakinra (Kineret, Swedish Orphan Biovitrum), would quench the acute inflammatory response in patients with ADHF.
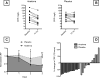
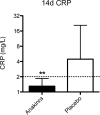
Methods: We randomized 30 patients with ADHF, reduced left ventricular ejection fraction (<40%), and elevated C reactive protein (CRP) levels (≥5 mg/L) to either anakinra 100 mg twice daily for 3 days followed by once daily for 11 days or matching placebo, in a 1:1 double blinded fashion. We measured daily CRP plasma levels using a high-sensitivity assay during hospitalization and then again at 14 days and evaluated the area-under-the-curve and interval changes (delta).
Results: Treatment with anakinra was well tolerated. At 72 hours, anakinra reduced CRP by 61% versus baseline, compared with a 6% reduction among patients receiving placebo (P = 0.004 anakinra vs. placebo).
Conclusions: Interleukin-1 blockade with anakinra reduces the systemic inflammatory response in patients with ADHF. Further studies are warranted to determine whether this anti-inflammatory effect translates into improved clinical outcomes.
Figures

References
-
- Weintraub NL, Collins SP, Pang PS, Levy PD, Anderson AS, Arslanian-Engoren C, Gibler WB, McCord JK, Parshall MB, Francis GS, Gheorghiade M. Acute heart failure syndromes: emergency department presentation, treatment, and disposition: current approaches and future aims: a scientific statement from the American Heart Association. Circulation. 2010;122:1975–96. - PubMed
-
- Gwadry-Sridhar FH, Flintoft V, Lee DS, Lee H, Guyatt GH. A systematic review and meta-analysis of studies comparing readmission rates and mortality rates in patients with heart failure. Arch Intern Med. 2004;164:2315–20. - PubMed
-
- Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–28. - PubMed
-
- Keenan PS, Normand SL, Lin Z, Drye EE, Bhat KR, Ross JS, Schuur JD, Stauffer BD, Bernheim SM, Epstein AJ, Wang Y, Herrin J, Chen J, Federer JJ, Mattera JA, Wang Y, Krumholz HM. An administrative claims measure suitable for profiling hospital performance on the basis of 30-day all-cause readmission rates among patients with heart failure. Circ Cardiovasc Qual Outcomes. 2008;1:29–37. - PubMed
-
- Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA. 2002;287:1531–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

