Temporal control of bidirectional lipid-droplet motion in Drosophila depends on the ratio of kinesin-1 and its co-factor Halo
- PMID: 26906417
- PMCID: PMC4852724
- DOI: 10.1242/jcs.183426
Temporal control of bidirectional lipid-droplet motion in Drosophila depends on the ratio of kinesin-1 and its co-factor Halo
Abstract
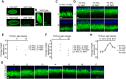
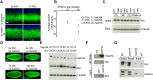
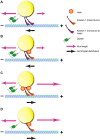
During bidirectional transport, individual cargoes move continuously back and forth along microtubule tracks, yet the cargo population overall displays directed net transport. How such transport is controlled temporally is not well understood. We analyzed this issue for bidirectionally moving lipid droplets in Drosophila embryos, a system in which net transport direction is developmentally controlled. By quantifying how the droplet distribution changes as embryos develop, we characterize temporal transitions in net droplet transport and identify the crucial contribution of the previously identified, but poorly characterized, transacting regulator Halo. In particular, we find that Halo is transiently expressed; rising and falling Halo levels control the switches in global distribution. Rising Halo levels have to pass a threshold before net plus-end transport is initiated. This threshold level depends on the amount of the motor kinesin-1: the more kinesin-1 is present, the more Halo is needed before net plus-end transport commences. Because Halo and kinesin-1 are present in common protein complexes, we propose that Halo acts as a rate-limiting co-factor of kinesin-1.
Keywords: Bidirectional transport; Drosophila embryos; Kinesin-1; Lipid droplets.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

