Mechanisms of Cardiac Regeneration
- PMID: 26906733
- PMCID: PMC4768311
- DOI: 10.1016/j.devcel.2016.01.018
Mechanisms of Cardiac Regeneration
Abstract
Adult humans fail to regenerate their hearts following injury, and this failure to regenerate myocardium is a leading cause of heart failure and death worldwide. Although all adult mammals appear to lack significant cardiac regeneration potential, some vertebrates can regenerate myocardium throughout life. In addition, new studies indicate that mammals have cardiac regeneration potential during development and very soon after birth. The mechanisms of heart regeneration among model organisms, including neonatal mice, appear remarkably similar. Orchestrated waves of inflammation, matrix deposition and remodeling, and cardiomyocyte proliferation are commonly seen in heart regeneration models. Understanding why adult mammals develop extensive scarring instead of regeneration is a crucial goal for regenerative biology.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures

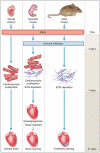
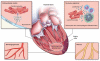
References
-
- Azcoitia V, Aracil M, Martínez-A C, Torres M. The homeodomain protein Meis1 is essential for definitive hematopoiesis and vascular patterning in the mouse embryo. Dev. Biol. 2005;280:307–320. - PubMed
-
- Baliga RR, Pimental DR, Zhao YY, Simmons WW, Marchionni MA, Sawyer DB, Kelly RA. NRG-1-induced cardiomyocyte hypertrophy. Role of PI-3-kinase, p70(S6K), and MEK-MAPK-RSK. Am. J. Physiol. 1999;277:H2026–H2037. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

