Recent Advances in Disease Modeling and Drug Discovery for Diabetes Mellitus Using Induced Pluripotent Stem Cells
- PMID: 26907255
- PMCID: PMC4783985
- DOI: 10.3390/ijms17020256
Recent Advances in Disease Modeling and Drug Discovery for Diabetes Mellitus Using Induced Pluripotent Stem Cells
Abstract
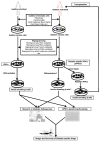
Diabetes mellitus (DM) is a widespread metabolic disease with a progressive incidence of morbidity and mortality worldwide. Despite extensive research, treatment options for diabetic patients remains limited. Although significant challenges remain, induced pluripotent stem cells (iPSCs) have the capacity to differentiate into any cell type, including insulin-secreting pancreatic β cells, highlighting its potential as a treatment option for DM. Several iPSC lines have recently been derived from both diabetic and healthy donors. Using different reprogramming techniques, iPSCs were differentiated into insulin-secreting pancreatic βcells. Furthermore, diabetes patient-derived iPSCs (DiPSCs) are increasingly being used as a platform to perform cell-based drug screening in order to develop DiPSC-based cell therapies against DM. Toxicity and teratogenicity assays based on iPSC-derived cells can also provide additional information on safety before advancing drugs to clinical trials. In this review, we summarize recent advances in the development of techniques for differentiation of iPSCs or DiPSCs into insulin-secreting pancreatic β cells, their applications in drug screening, and their role in complementing and replacing animal testing in clinical use. Advances in iPSC technologies will provide new knowledge needed to develop patient-specific iPSC-based diabetic therapies.
Keywords: cell-based drug screening; diabetes mellitus; iPSC-based diabetic therapy; induced pluripotent stem cells; insulin-secreting β cells.
Figures

References
-
- Defronzo R.A. Pathogenesis of type 2 diabetes: Metabolic and molecular implications for identifying diabetes genes. Diabetes Rev. 1997;5:177–269. doi: 10.2165/00003495-199958001-00008. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

