Chemical Diversity and Biological Properties of Secondary Metabolites from Sea Hares of Aplysia Genus
- PMID: 26907303
- PMCID: PMC4771992
- DOI: 10.3390/md14020039
Chemical Diversity and Biological Properties of Secondary Metabolites from Sea Hares of Aplysia Genus
Abstract
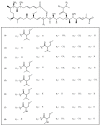
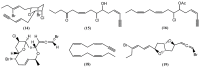
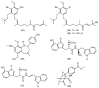
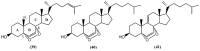
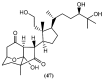
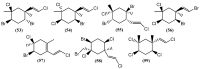
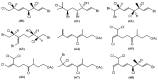
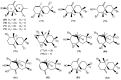
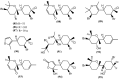
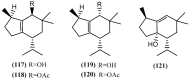
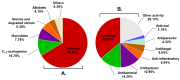
The marine environment is an important source of structurally-diverse and biologically-active secondary metabolites. During the last two decades, thousands of compounds were discovered in marine organisms, several of them having inspired the development of new classes of therapeutic agents. Marine mollusks constitute a successful phyla in the discovery of new marine natural products (MNPs). Over a 50-year period from 1963, 116 genera of mollusks contributed innumerous compounds, Aplysia being the most studied genus by MNP chemists. This genus includes 36 valid species and should be distinguished from all mollusks as it yielded numerous new natural products. Aplysia sea hares are herbivorous mollusks, which have been proven to be a rich source of secondary metabolites, mostly of dietary origin. The majority of secondary metabolites isolated from sea hares of the genus Aplysia are halogenated terpenes; however, these animals are also a source of compounds from other chemical classes, such as macrolides, sterols and alkaloids, often exhibiting cytotoxic, antibacterial, antifungal, antiviral and/or antifeedant activities. This review focuses on the diverse structural classes of secondary metabolites found in Aplysia spp., including several compounds with pronounced biological properties.
Keywords: Aplysia; biological properties; mollusks; sea hares; secondary metabolites.
Figures




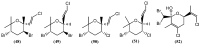
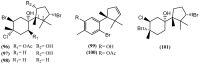







Similar articles
-
Halogenated Metabolites from the Diet of Aplysia dactylomela Rang.Molecules. 2020 Feb 13;25(4):815. doi: 10.3390/molecules25040815. Molecules. 2020. PMID: 32070000 Free PMC article. Review.
-
Pharmaceutically active secondary metabolites of marine actinobacteria.Microbiol Res. 2014 Apr;169(4):262-78. doi: 10.1016/j.micres.2013.07.014. Epub 2013 Aug 16. Microbiol Res. 2014. PMID: 23958059 Review.
-
New Perspectives in the Chemistry of Marine Pyridoacridine Alkaloids.Mar Drugs. 2016 Jan 26;14(2):26. doi: 10.3390/md14020026. Mar Drugs. 2016. PMID: 26821033 Free PMC article. Review.
-
The Laurencia Paradox: An Endless Source of Chemodiversity.Prog Chem Org Nat Prod. 2016;102:91-252. doi: 10.1007/978-3-319-33172-0_2. Prog Chem Org Nat Prod. 2016. PMID: 27380407 Review.
-
Bioactive Compounds Produced by Strains of Penicillium and Talaromyces of Marine Origin.Mar Drugs. 2016 Feb 18;14(2):37. doi: 10.3390/md14020037. Mar Drugs. 2016. PMID: 26901206 Free PMC article. Review.
Cited by
-
Halogenated Metabolites from the Diet of Aplysia dactylomela Rang.Molecules. 2020 Feb 13;25(4):815. doi: 10.3390/molecules25040815. Molecules. 2020. PMID: 32070000 Free PMC article. Review.
-
Construction of Seven-Membered Oxacycles Using a Rh(I)-Catalyzed Cascade C-C Formation/Cleavage of Cyclobutenol Derivatives.J Org Chem. 2024 Apr 5;89(7):4647-4656. doi: 10.1021/acs.joc.3c02914. Epub 2024 Mar 18. J Org Chem. 2024. PMID: 38497619 Free PMC article.
-
Discovery of Anti-MRSA Secondary Metabolites from a Marine-Derived Fungus Aspergillus fumigatus.Mar Drugs. 2022 Apr 28;20(5):302. doi: 10.3390/md20050302. Mar Drugs. 2022. PMID: 35621953 Free PMC article.
-
Sea Hare Hydrolysate-Induced Reduction of Human Non-Small Cell Lung Cancer Cell Growth through Regulation of Macrophage Polarization and Non-Apoptotic Regulated Cell Death Pathways.Cancers (Basel). 2020 Mar 19;12(3):726. doi: 10.3390/cancers12030726. Cancers (Basel). 2020. PMID: 32204484 Free PMC article.
-
Molluscan Compounds Provide Drug Leads for the Treatment and Prevention of Respiratory Disease.Mar Drugs. 2020 Nov 19;18(11):570. doi: 10.3390/md18110570. Mar Drugs. 2020. PMID: 33228163 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

