IκBα mediates prostate cancer cell death induced by combinatorial targeting of the androgen receptor
- PMID: 26907477
- PMCID: PMC4785192
- DOI: 10.1186/s12885-016-2188-2
IκBα mediates prostate cancer cell death induced by combinatorial targeting of the androgen receptor
Erratum in
-
Erratum to: IκBα mediates prostate cancer cell death induced by combinatorial targeting of the androgen receptor.BMC Cancer. 2016 Mar 3;16:177. doi: 10.1186/s12885-016-2215-3. BMC Cancer. 2016. PMID: 26940599 Free PMC article. No abstract available.
Abstract
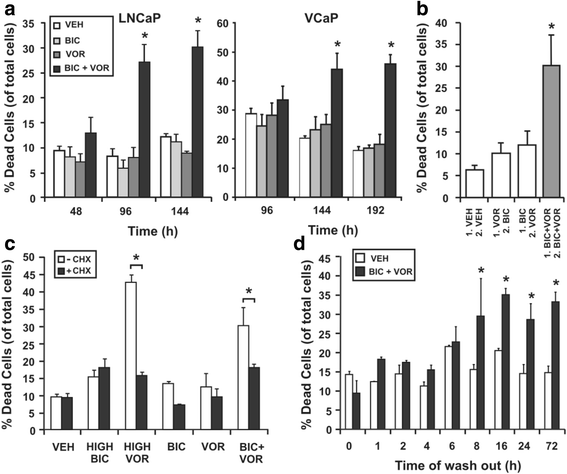
Background: Combining different clinical agents to target multiple pathways in prostate cancer cells, including androgen receptor (AR) signaling, is potentially an effective strategy to improve outcomes for men with metastatic disease. We have previously demonstrated that sub-effective concentrations of an AR antagonist, bicalutamide, and the histone deacetylase inhibitor, vorinostat, act synergistically when combined to cause death of AR-dependent prostate cancer cells.
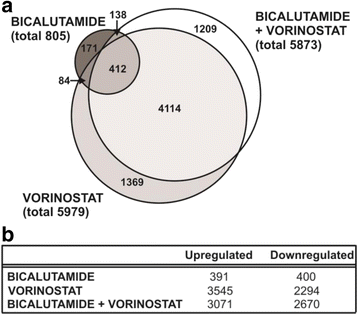
Methods: In this study, expression profiling of human prostate cancer cells treated with bicalutamide or vorinostat, alone or in combination, was employed to determine the molecular mechanisms underlying this synergistic action. Cell viability assays and quantitative real time PCR were used to validate identified candidate genes.
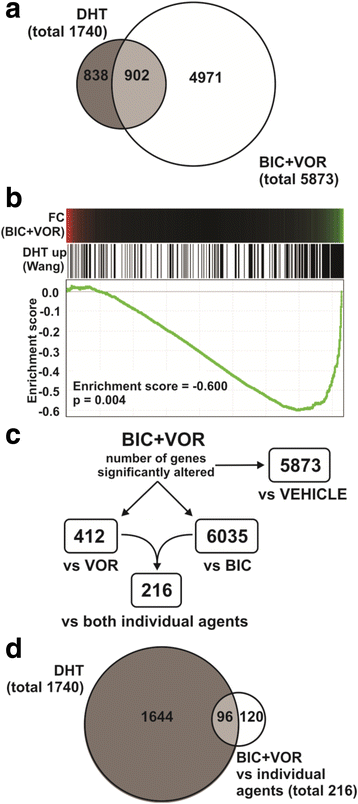
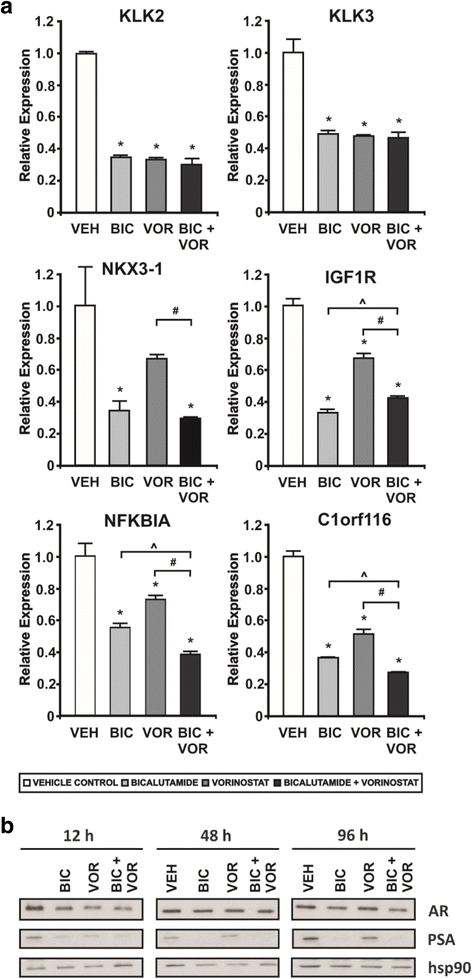
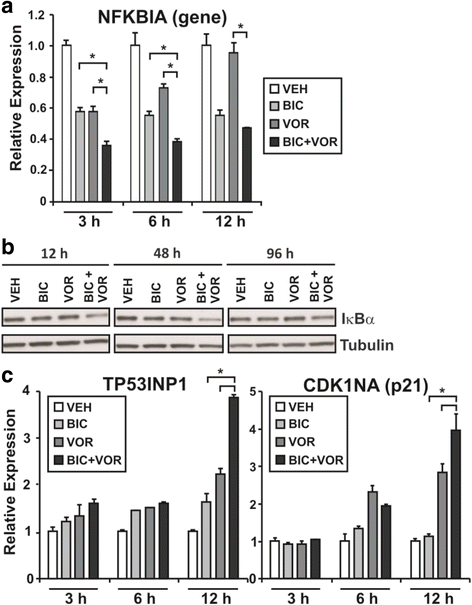
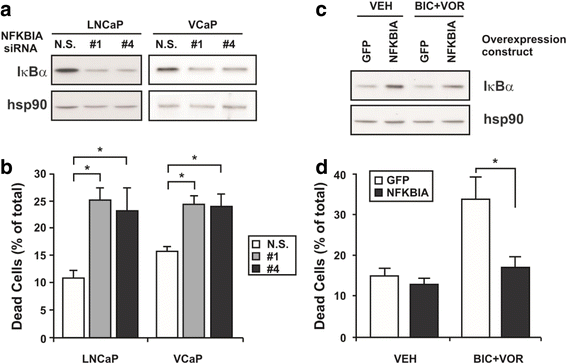
Results: A substantial proportion of the genes modulated by the combination of bicalutamide and vorinostat were androgen regulated. Independent pathway analysis identified further pathways and genes, most notably NFKBIA (encoding IκBα, an inhibitor of NF-κB and p53 signaling), as targets of this combinatorial treatment. Depletion of IκBα by siRNA knockdown enhanced apoptosis of prostate cancer cells, while ectopic overexpression of IκBα markedly suppressed cell death induced by the combination of bicalutamide and vorinostat.
Conclusion: These findings implicate IκBα as a key mediator of the apoptotic action of this combinatorial AR targeting strategy and a promising new therapeutic target for prostate cancer.
Figures
Similar articles
-
Suberoylanilide hydroxamic acid (vorinostat) represses androgen receptor expression and acts synergistically with an androgen receptor antagonist to inhibit prostate cancer cell proliferation.Mol Cancer Ther. 2007 Jan;6(1):51-60. doi: 10.1158/1535-7163.MCT-06-0144. Epub 2007 Jan 11. Mol Cancer Ther. 2007. PMID: 17218635
-
Metformin represses androgen-dependent and androgen-independent prostate cancers by targeting androgen receptor.Prostate. 2015 Aug 1;75(11):1187-96. doi: 10.1002/pros.23000. Epub 2015 Apr 20. Prostate. 2015. PMID: 25894097
-
Induction of bicalutamide sensitivity in prostate cancer cells by an epigenetic Puralpha-mediated decrease in androgen receptor levels.Prostate. 2010 Feb 1;70(2):179-89. doi: 10.1002/pros.21051. Prostate. 2010. PMID: 19790234
-
Defining the molecular action of HDAC inhibitors and synergism with androgen deprivation in ERG-positive prostate cancer.Int J Cancer. 2008 Dec 15;123(12):2774-81. doi: 10.1002/ijc.23885. Int J Cancer. 2008. PMID: 18798265
-
5-Azacitidine restores and amplifies the bicalutamide response on preclinical models of androgen receptor expressing or deficient prostate tumors.Prostate. 2010 Aug;70(11):1166-78. doi: 10.1002/pros.21151. Prostate. 2010. PMID: 20333699
Cited by
-
A novel computational approach for drug repurposing using systems biology.Bioinformatics. 2018 Aug 15;34(16):2817-2825. doi: 10.1093/bioinformatics/bty133. Bioinformatics. 2018. PMID: 29534151 Free PMC article.
-
Delineation of the androgen-regulated signaling pathways in prostate cancer facilitates the development of novel therapeutic approaches.Curr Opin Pharmacol. 2018 Aug;41:1-11. doi: 10.1016/j.coph.2018.03.002. Epub 2018 Mar 30. Curr Opin Pharmacol. 2018. PMID: 29609138 Free PMC article. Review.
-
Erratum to: IκBα mediates prostate cancer cell death induced by combinatorial targeting of the androgen receptor.BMC Cancer. 2016 Mar 3;16:177. doi: 10.1186/s12885-016-2215-3. BMC Cancer. 2016. PMID: 26940599 Free PMC article. No abstract available.
-
Androgen Receptor-Mediated Transcription in Prostate Cancer.Cells. 2022 Mar 5;11(5):898. doi: 10.3390/cells11050898. Cells. 2022. PMID: 35269520 Free PMC article. Review.
-
Oridonin increases anticancer effects of lentinan in HepG2 human hepatoblastoma cells.Oncol Lett. 2018 Feb;15(2):1999-2005. doi: 10.3892/ol.2017.7485. Epub 2017 Nov 24. Oncol Lett. 2018. PMID: 29434900 Free PMC article.
References
-
- Huggins C, Stevens RE, Jr, Hodges CV. The effect of castration on advanced carcinoma of the prostate gland. Arch Surg. 1941;43:209. doi: 10.1001/archsurg.1941.01210140043004. - DOI
-
- Koivisto P, Kononen J, Palmberg C, Tammela T, Hyytinen E, Isola J, Trapman J, Cleutjens K, Noordzij A, Visakorpi T, et al. Androgen receptor gene amplification: a possible molecular mechanism for androgen deprivation therapy failure in prostate cancer. Cancer Res. 1997;57(2):314–319. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

