Disorders of Vascular Permeability
- PMID: 26907525
- PMCID: PMC8462517
- DOI: 10.1146/annurev-pathol-012615-044506
Disorders of Vascular Permeability
Abstract
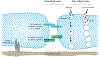
The endothelial barrier maintains vascular and tissue homeostasis and modulates many physiological processes, such as angiogenesis. Vascular barrier integrity can be disrupted by a variety of soluble permeability factors, and changes in barrier function can exacerbate tissue damage during disease progression. Understanding endothelial barrier function is critical for vascular homeostasis. Many of the signaling pathways promoting vascular permeability can also be triggered during disease, resulting in prolonged or uncontrolled vascular leak. It is believed that recovery of the normal vasculature requires diminishing this hyperpermeable state. Although the molecular mechanisms governing vascular leak have been studied over the last few decades, recent advances have identified new therapeutic targets that have begun to show preclinical and clinical promise. These approaches have been successfully applied to an increasing number of disease conditions. New perspectives regarding how vascular leak impacts the progression of various diseases are highlighted in this review.
Keywords: angiogenesis; barrier; endothelium; junctions; permeability.
Figures


References
-
- Mehta D, Malik AB. 2006. Signaling mechanisms regulating endothelial permeability. Physiol. Rev 86:279–367 - PubMed
-
- Folkman J 1995. Seminars in Medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. N. Engl. J. Med 333:1757–63 - PubMed
-
- Carmeliet P 2005. Angiogenesis in life, disease and medicine. Nature 438:932–36 - PubMed
-
- Folkman J 2007. Angiogenesis: an organizing principle for drug discovery? Nat. Rev. Drug Discov 6:273–86 - PubMed
-
- Folkman J 2003. Fundamental concepts of the angiogenic process. Curr. Mol. Med 3:643–51 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

