Constitutively Elevated Blood Serotonin Is Associated with Bone Loss and Type 2 Diabetes in Rats
- PMID: 26907598
- PMCID: PMC4764355
- DOI: 10.1371/journal.pone.0150102
Constitutively Elevated Blood Serotonin Is Associated with Bone Loss and Type 2 Diabetes in Rats
Abstract
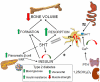
Reduced peripheral serotonin (5HT) in mice lacking tryptophan hydroxylase (TPH1), the rate limiting enzyme for 5HT synthesis, was reported to be anabolic to the skeleton. However, in other studies TPH1 deletion either had no bone effect or an age dependent inhibition of osteoclastic bone resorption. The role of 5HT in bone therefore remains poorly understood. To address this issue, we used selective breeding to create rat sublines with constitutively high (high-5HT) and low (low-5HT) platelet 5HT level (PSL) and platelet 5HT uptake (PSU). High-5HT rats had decreased bone volume due to increased bone turnover characterized by increased bone formation and mineral apposition rate, increased osteoclast number and serum C-telopeptide level. Daily oral administration of the TPH1 inhibitor (LX1032) for 6 weeks reduced PSL and increased the trabecular bone volume and trabecular number of the spine and femur in high-5HT rats. High-5HT animals also developed a type 2 diabetes (T2D) phenotype with increased: plasma insulin, glucose, hemoglobin A1c, body weight, visceral fat, β-cell pancreatic islets size, serum cholesterol, and decreased muscle strength. Serum calcium accretion mediated by parathyroid hormone slightly increased, whereas treatment with 1,25(OH)2D3 decreased PSL. Insulin reduction was paralleled by a drop in PSL in high-5HT rats. In vitro, insulin and 5HT synergistically up-regulated osteoblast differentiation isolated from high-5HT rats, whereas TPH1 inhibition decreased the number of bone marrow-derived osteoclasts. These results suggest that constitutively elevated PSL is associated with bone loss and T2D via a homeostatic interplay between the peripheral 5HT, bone and insulin.
Conflict of interest statement
Figures








References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

