Development of Spexin-based Human Galanin Receptor Type II-Specific Agonists with Increased Stability in Serum and Anxiolytic Effect in Mice
- PMID: 26907960
- PMCID: PMC4764904
- DOI: 10.1038/srep21453
Development of Spexin-based Human Galanin Receptor Type II-Specific Agonists with Increased Stability in Serum and Anxiolytic Effect in Mice
Abstract
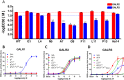
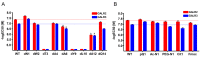
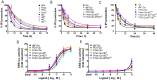
The novel neuropeptide spexin (SPX) was discovered to activate galanin receptor 2 (GALR2) and 3 (GALR3) but not galanin receptor 1 (GALR1). Although GALR2 is known to display a function, particularly in anxiety, depression, and appetite regulation, the further determination of its function would benefit from a more stable and selective agonist that acts only at GALR2. In the present study, we developed a GALR2-specific agonist with increased stability in serum. As galanin (GAL) showed a low affinity to GALR3, the residues in SPX were replaced with those in GAL, revealing that particular mutations such as Gln5 → Asn, Met7 → Ala, Lys11 → Phe, and Ala13 → Pro significantly decreased potencies toward GALR3 but not toward GALR2. Quadruple (Qu) mutation of these residues still retained potency to GALR2 but totally abolished the potency to both GALR3 and GALR1. The first amino acid modifications or D-Asn1 substitution significantly increased the stability when they are incubated in 100% fetal bovine serum. Intracerebroventricular administration of the mutant peptide with D-Asn1 and quadruple substitution (dN1-Qu) exhibited an anxiolytic effect in mice. Taken together, the GALR2-specific agonist with increased stability can greatly help delineation of GALR2-mediated functions and be very useful for treatments of anxiety disorder.
Figures


References
-
- Wan B. et al. C120RF39, a novel secreted protein with a typical amidation processing signal. Biosci Rep 30, 1–10 (2010). - PubMed
-
- Liu Y. et al. A novel neuropeptide in suppresing luteinizing hormone release in goldfish, Carassius auratus. Mol Cell Endocrinol 374, 65–72 (2013). - PubMed
-
- Wong M. K. et al. Goldfish spexin: solution structure and novel function as a satiety factor in feeding control. Am J Physiol Endocrinol Metab 305, E348–366 (2013). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

