Microneedles: A New Frontier in Nanomedicine Delivery
- PMID: 26908048
- PMCID: PMC4820498
- DOI: 10.1007/s11095-016-1885-5
Microneedles: A New Frontier in Nanomedicine Delivery
Abstract
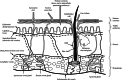
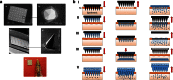
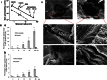
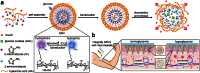
This review aims to concisely chart the development of two individual research fields, namely nanomedicines, with specific emphasis on nanoparticles (NP) and microparticles (MP), and microneedle (MN) technologies, which have, in the recent past, been exploited in combinatorial approaches for the efficient delivery of a variety of medicinal agents across the skin. This is an emerging and exciting area of pharmaceutical sciences research within the remit of transdermal drug delivery and as such will undoubtedly continue to grow with the emergence of new formulation and fabrication methodologies for particles and MN. Firstly, the fundamental aspects of skin architecture and structure are outlined, with particular reference to their influence on NP and MP penetration. Following on from this, a variety of different particles are described, as are the diverse range of MN modalities currently under development. The review concludes by highlighting some of the novel delivery systems which have been described in the literature exploiting these two approaches and directs the reader towards emerging uses for nanomedicines in combination with MN.
Keywords: drug delivery; microneedles; microparticles; nanomedicine; nanoparticles; vaccines.
Figures





References
-
- Parveen S, Misra R, Sahoo SK. Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging. Nanomedicine. 2012;8(2):147–66. - PubMed
-
- Patravale V, Dandekar P, Jain R. Nanoparticulate drug delivery: perspectives on the transition from laboratory to market: Elsevier Science; 2012.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

