Hepatic macrophages in homeostasis and liver diseases: from pathogenesis to novel therapeutic strategies
- PMID: 26908374
- PMCID: PMC4856798
- DOI: 10.1038/cmi.2015.104
Hepatic macrophages in homeostasis and liver diseases: from pathogenesis to novel therapeutic strategies
Abstract
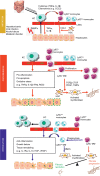
Macrophages represent a major cell type of innate immunity and have emerged as a critical player and therapeutic target in many chronic inflammatory diseases. Hepatic macrophages consist of Kupffer cells, which are originated from the fetal yolk-sack, and infiltrated bone marrow-derived monocytes/macrophages. Hepatic macrophages play a central role in maintaining homeostasis of the liver and in the pathogenesis of liver injury, making them an attractive therapeutic target for liver diseases. However, the various populations of hepatic macrophages display different phenotypes and exert distinct functions. Thus, more research is required to better understand these cells to guide the development of macrophage-based therapeutic interventions. This review article will summarize the current knowledge on the origins and composition of hepatic macrophages, their functions in maintaining hepatic homeostasis, and their involvement in both promoting and resolving liver inflammation, injury, and fibrosis. Finally, the current strategies being developed to target hepatic macrophages for the treatment of liver diseases will be reviewed.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

