Mutations in POMGNT1 cause non-syndromic retinitis pigmentosa
- PMID: 26908613
- PMCID: PMC4805308
- DOI: 10.1093/hmg/ddw022
Mutations in POMGNT1 cause non-syndromic retinitis pigmentosa
Abstract
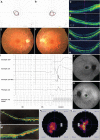
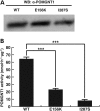
A growing number of human diseases have been linked to defects in protein glycosylation that affects a wide range of organs. Among them, O-mannosylation is an unusual type of protein glycosylation that is largely restricted to the muscular and nerve system. Consistently, mutations in genes involved in the O-mannosylation pathway result in infantile-onset, severe developmental defects involving skeleton muscle, brain and eye, such as the muscle-eye-brain disease (MIM no. 253280). However, the functional importance of O-mannosylation in these tissues at later stages remains largely unknown. In our study, we have identified recessive mutations in POMGNT1, which encodes an essential component in O-mannosylation pathway, in three unrelated families with autosomal recessive retinitis pigmentosa (RP), but without extraocular involvement. Enzymatic assay of these mutant alleles demonstrate that they greatly reduce the POMGNT1 enzymatic activity and are likely to be hypomorphic. Immunohistochemistry shows that POMGNT1 is specifically expressed in photoreceptor basal body. Taken together, our work identifies a novel disease-causing gene for RP and indicates that proper protein O-mannosylation is not only essential for early organ development, but also important for maintaining survival and function of the highly specialized retinal cells at later stages.
© The Author 2016. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures


References
-
- Wang F., Wang H., Tuan H.F., Nguyen D.H., Sun V., Keser V., Bowne S.J., Sullivan L.S., Luo H., Zhao L. et al. (2014) Next generation sequencing-based molecular diagnosis of retinitis pigmentosa: identification of a novel genotype-phenotype correlation and clinical refinements. Hum. Genet., 133, 331–345. - PMC - PubMed
-
- Haltia M. (2006) The neuronal ceroid-lipofuscinoses: from past to present. Biochim. Biophys. Acta, 1762, 850–856. - PubMed
-
- Hayflick S.J. (2003) Unraveling the Hallervorden-Spatz syndrome: pantothenate kinase-associated neurodegeneration is the name. Curr. Opin. Pediatr., 15, 572–577. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

