Identification of Parvalbumin Interneurons as Cellular Substrate of Fear Memory Persistence
- PMID: 26908632
- PMCID: PMC4830301
- DOI: 10.1093/cercor/bhw001
Identification of Parvalbumin Interneurons as Cellular Substrate of Fear Memory Persistence
Abstract
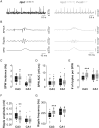
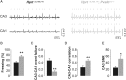
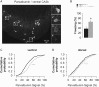
Parvalbumin-positive (PV) basket cells provide perisomatic inhibition in the cortex and hippocampus and control generation of memory-related network activity patterns, such as sharp wave ripples (SPW-R). Deterioration of this class of fast-spiking interneurons has been observed in neuropsychiatric disorders and evidence from animal models suggests their involvement in the acquisition and extinction of fear memories. Here, we used mice with neuron type-targeted expression of the presynaptic gain-of-function glycine receptor RNA variant GlyR α3L(185L)to genetically enhance the network activity of PV interneurons. These mice showed reduced extinction of contextual fear memory but normal auditory cued fear memory. They furthermore displayed increase of SPW-R activity in area CA3 and CA1 and facilitated propagation of this particular network activity pattern, as determined in ventral hippocampal slice preparations. Individual freezing levels during extinction and SPW-R propagation were correlated across genotypes. The same was true for parvalbumin immunoreactivity in the ventral hippocampus, which was generally augmented in the GlyR mutant mice and correlated with individual freezing levels. Together, these results identify PV interneurons as critical cellular substrate of fear memory persistence and associated SPW-R activity in the hippocampus. Our findings may be relevant for the identification and characterization of physiological correlates for posttraumatic stress and anxiety disorders.
Keywords: contextual fear memory; hippocampus; interneurons; memory extinction; parvalbumin; sharp wave ripples.
© The Author 2016. Published by Oxford University Press.
Figures




References
-
- Albrecht A, Bergado-Acosta JR, Pape HC, Stork O. 2010. Role of the neural cell adhesion molecule (NCAM) in amygdalo-hippocampal interactions and salience determination of contextual fear memory. Int J Neuropsychopharmacol. 13:661–674. - PubMed
-
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J. 2004. Regional dissociations within the hippocampus--memory and anxiety. Neurosci Biobehav Rev. 28:273–283. - PubMed
-
- Bannerman DM, Sprengel R, Sanderson DJ, McHugh SB, Rawlins JN, Monyer H, Seeburg PH. 2014. Hippocampal synaptic plasticity, spatial memory and anxiety. Nat Rev Neurosci. 15:181–192. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

