A DNA binding winged helix domain in CAF-1 functions with PCNA to stabilize CAF-1 at replication forks
- PMID: 26908650
- PMCID: PMC4914081
- DOI: 10.1093/nar/gkw106
A DNA binding winged helix domain in CAF-1 functions with PCNA to stabilize CAF-1 at replication forks
Abstract
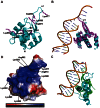
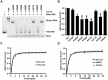
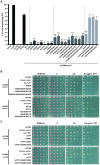
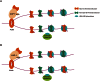
Chromatin assembly factor 1 (CAF-1) is a histone H3-H4 chaperone that deposits newly synthesized histone (H3-H4)2 tetramers during replication-coupled nucleosome assembly. However, how CAF-1 functions in this process is not yet well understood. Here, we report the crystal structure of C terminus of Cac1 (Cac1C), a subunit of yeast CAF-1, and the function of this domain in stabilizing CAF-1 at replication forks. We show that Cac1C forms a winged helix domain (WHD) and binds DNA in a sequence-independent manner. Mutations in Cac1C that abolish DNA binding result in defects in transcriptional silencing and increased sensitivity to DNA damaging agents, and these defects are exacerbated when combined with Cac1 mutations deficient in PCNA binding. Similar phenotypes are observed for corresponding mutations in mouse CAF-1. These results reveal a mechanism conserved in eukaryotic cells whereby the ability of CAF-1 to bind DNA is important for its association with the DNA replication forks and subsequent nucleosome assembly.
© The Author(s) 2016. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


Similar articles
-
Structural Basis for the Interaction Between Yeast Chromatin Assembly Factor 1 and Proliferating Cell Nuclear Antigen.J Mol Biol. 2024 Aug 15;436(16):168695. doi: 10.1016/j.jmb.2024.168695. Epub 2024 Jul 4. J Mol Biol. 2024. PMID: 38969056 Free PMC article.
-
The Cac2 subunit is essential for productive histone binding and nucleosome assembly in CAF-1.Sci Rep. 2017 Apr 18;7:46274. doi: 10.1038/srep46274. Sci Rep. 2017. PMID: 28418026 Free PMC article.
-
Two fundamentally distinct PCNA interaction peptides contribute to chromatin assembly factor 1 function.Mol Cell Biol. 2009 Dec;29(24):6353-65. doi: 10.1128/MCB.01051-09. Epub 2009 Oct 12. Mol Cell Biol. 2009. PMID: 19822659 Free PMC article.
-
Mechanistic insights into histone deposition and nucleosome assembly by the chromatin assembly factor-1.Nucleic Acids Res. 2018 Nov 2;46(19):9907-9917. doi: 10.1093/nar/gky823. Nucleic Acids Res. 2018. PMID: 30239791 Free PMC article. Review.
-
The ins and outs of nucleosome assembly.Curr Opin Genet Dev. 2001 Apr;11(2):136-41. doi: 10.1016/s0959-437x(00)00170-2. Curr Opin Genet Dev. 2001. PMID: 11250135 Review.
Cited by
-
Identification of Elg1 interaction partners and effects on post-replication chromatin re-formation.PLoS Genet. 2018 Nov 12;14(11):e1007783. doi: 10.1371/journal.pgen.1007783. eCollection 2018 Nov. PLoS Genet. 2018. PMID: 30418970 Free PMC article.
-
Pivotal roles of PCNA loading and unloading in heterochromatin function.Proc Natl Acad Sci U S A. 2018 Feb 27;115(9):E2030-E2039. doi: 10.1073/pnas.1721573115. Epub 2018 Feb 13. Proc Natl Acad Sci U S A. 2018. PMID: 29440488 Free PMC article.
-
Structural Basis for the Interaction Between Yeast Chromatin Assembly Factor 1 and Proliferating Cell Nuclear Antigen.J Mol Biol. 2024 Aug 15;436(16):168695. doi: 10.1016/j.jmb.2024.168695. Epub 2024 Jul 4. J Mol Biol. 2024. PMID: 38969056 Free PMC article.
-
Asymmetric Histone Inheritance: Establishment, Recognition, and Execution.Annu Rev Genet. 2022 Nov 30;56:113-143. doi: 10.1146/annurev-genet-072920-125226. Epub 2022 Jul 29. Annu Rev Genet. 2022. PMID: 35905975 Free PMC article. Review.
-
The Role of Histone Modification in DNA Replication-Coupled Nucleosome Assembly and Cancer.Int J Mol Sci. 2023 Mar 3;24(5):4939. doi: 10.3390/ijms24054939. Int J Mol Sci. 2023. PMID: 36902370 Free PMC article. Review.
References
-
- Luger K., Mader A.W., Richmond R.K., Sargent D.F., Richmond T.J. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389:251–260. - PubMed
-
- Davey C.A., Sargent D.F., Luger K., Maeder A.W., Richmond T.J. Solvent mediated interactions in the structure of the nucleosome core particle at 1.9 a resolution. J. Mol. Biol. 2002;319:1097–1113. - PubMed
-
- Vasudevan D., Chua E.Y., Davey C.A. Crystal structures of nucleosome core particles containing the ‘601’ strong positioning sequence. J. Mol. Biol. 2010;403:1–10. - PubMed
-
- Falbo K.B., Shen X. Chromatin remodeling in DNA replication. J. Cell. Biochem. 2006;97:684–689. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

