Preschool Assessment of Preterm Infants Treated With Darbepoetin and Erythropoietin
- PMID: 26908704
- PMCID: PMC4771132
- DOI: 10.1542/peds.2015-3859
Preschool Assessment of Preterm Infants Treated With Darbepoetin and Erythropoietin
Abstract
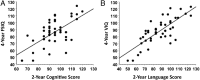
Background: We previously reported improved neurodevelopmental outcomes at 2 years among infants treated with the erythropoiesis-stimulating agents (ESAs) darbepoetin alfa (darbepoetin) or erythropoietin. Here we characterize 4-year outcomes.
Methods: Former preterm infants randomly assigned to receive darbepoetin (10 μg/kg, once per week), erythropoietin (400 U/kg, 3 times/week), or placebo through 35 weeks' postconceptual age were evaluated at 3.5 to 4 years of age. For comparison, healthy children formerly delivered full term (term controls [TCs]) were also recruited. All participants were assessed by using measures of full-scale IQ (FSIQ) and general language from the Wechsler Preschool and Primary Scale of Intelligence, Third Edition, and an overall measure of executive function, on the basis of tests evaluating inhibitory control and spatial working memory. Rates of neurodevelopmental impairment were compared across groups.
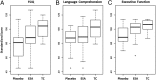
Results: Multivariate analysis of variance compared children randomly assigned to ESAs (n = 39), placebo (n =14), and TCs (n = 24). FSIQ and performance IQ were significantly higher in the ESA group than in the placebo group (FSIQ: 91.1 ± 17.5 vs 79.2 ± 18.5, P = .036; performance IQ: 93.0 ± 17.0 vs 79.5 ± 19.5, P = .018). Follow-up analyses revealed that the children receiving ESAs performed better than those who received placebo on executive function tasks. The ESA group's performance was below that of TCs, but the results did not reach significance on executive function. The incidence of neurodevelopmental impairment was greater in the placebo group than in the ESA group.
Conclusions: ESA-treated infants had better cognitive outcomes and less developmental impairment at 3.5 to 4 years of age compared with placebo-treated infants. ESAs show promise in improving long-term cognitive outcomes of infants born prematurely.
Trial registration: ClinicalTrials.gov NCT00334737 NCT01207778.
Copyright © 2016 by the American Academy of Pediatrics.
Conflict of interest statement
Figures
References
-
- Juul SE, Beyer RP, Bammler TK, McPherson RJ, Wilkerson J, Farin FM. Microarray analysis of high-dose recombinant erythropoietin treatment of unilateral brain injury in neonatal mouse hippocampus. Pediatr Res. 2009;65(5):485–492 - PubMed
-
- Chattopadhyay A, Choudhury TD, Bandyopadhyay D, Datta AG. Protective effect of erythropoietin on the oxidative damage of erythrocyte membrane by hydroxyl radical. Biochem Pharmacol. 2000;59(4):419–425 - PubMed
-
- Vairano M, Dello Russo C, Pozzoli G, et al. . Erythropoietin exerts anti-apoptotic effects on rat microglial cells in vitro. Eur J Neurosci. 2002;16(4):584–592 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

