Demonstration of the Blood-Stage Plasmodium falciparum Controlled Human Malaria Infection Model to Assess Efficacy of the P. falciparum Apical Membrane Antigen 1 Vaccine, FMP2.1/AS01
- PMID: 26908756
- PMCID: PMC4857475
- DOI: 10.1093/infdis/jiw039
Demonstration of the Blood-Stage Plasmodium falciparum Controlled Human Malaria Infection Model to Assess Efficacy of the P. falciparum Apical Membrane Antigen 1 Vaccine, FMP2.1/AS01
Erratum in
-
Payne et al (J Infect Dis 2016; 213:1743-51).J Infect Dis. 2016 Sep 15;214(6):978. doi: 10.1093/infdis/jiw202. Epub 2016 Jun 29. J Infect Dis. 2016. PMID: 27357343 Free PMC article. No abstract available.
Abstract
Background: Models of controlled human malaria infection (CHMI) initiated by mosquito bite have been widely used to assess efficacy of preerythrocytic vaccine candidates in small proof-of-concept phase 2a clinical trials. Efficacy testing of blood-stage malaria parasite vaccines, however, has generally relied on larger-scale phase 2b field trials in malaria-endemic populations. We report the use of a blood-stage P. falciparum CHMI model to assess blood-stage vaccine candidates, using their impact on the parasite multiplication rate (PMR) as the primary efficacy end point.
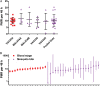
Methods: Fifteen healthy United Kingdom adult volunteers were vaccinated with FMP2.1, a protein vaccine that is based on the 3D7 clone sequence of apical membrane antigen 1 (AMA1) and formulated in Adjuvant System 01 (AS01). Twelve vaccinees and 15 infectivity controls subsequently underwent blood-stage CHMI. Parasitemia was monitored by quantitative real-time polymerase chain reaction (PCR) analysis, and PMR was modeled from these data.
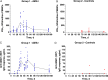
Results: FMP2.1/AS01 elicited anti-AMA1 T-cell and serum antibody responses. Analysis of purified immunoglobulin G showed functional growth inhibitory activity against P. falciparum in vitro. There were no vaccine- or CHMI-related safety concerns. All volunteers developed blood-stage parasitemia, with no impact of the vaccine on PMR.
Conclusions: FMP2.1/AS01 demonstrated no efficacy after blood-stage CHMI. However, the model induced highly reproducible infection in all volunteers and will accelerate proof-of-concept testing of future blood-stage vaccine candidates.
Clinical trials registration: NCT02044198.
Keywords: AMA1; CHMI; blood stage; malaria; vaccine.
© The Author 2016. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail journals.permissions@oup.com.
Figures


References
-
- Moorthy VS, Newman RD, Okwo-Bele JM. Malaria vaccine technology roadmap. Lancet 2013; 382:1700–1. - PubMed
-
- Halbroth BR, Draper SJ. Recent developments in malaria vaccinology. Adv Parasitol 2015; 88:1–49. - PubMed
-
- Goodman AL, Draper SJ. Blood-stage malaria vaccines - recent progress and future challenges. Ann Trop Med Parasitol 2010; 104:189–211. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

