An Ancient Bacterial Signaling Pathway Regulates Chloroplast Function to Influence Growth and Development in Arabidopsis
- PMID: 26908759
- PMCID: PMC4826016
- DOI: 10.1105/tpc.16.00045
An Ancient Bacterial Signaling Pathway Regulates Chloroplast Function to Influence Growth and Development in Arabidopsis
Abstract
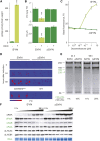
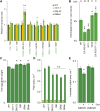
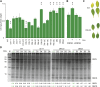
The chloroplast originated from the endosymbiosis of an ancient photosynthetic bacterium by a eukaryotic cell. Remarkably, the chloroplast has retained elements of a bacterial stress response pathway that is mediated by the signaling nucleotides guanosine penta- and tetraphosphate (ppGpp). However, an understanding of the mechanism and outcomes of ppGpp signaling in the photosynthetic eukaryotes has remained elusive. Using the model plant Arabidopsis thaliana, we show that ppGpp is a potent regulator of chloroplast gene expression in vivo that directly reduces the quantity of chloroplast transcripts and chloroplast-encoded proteins. We then go on to demonstrate that the antagonistic functions of different plant RelA SpoT homologs together modulate ppGpp levels to regulate chloroplast function and show that they are required for optimal plant growth, chloroplast volume, and chloroplast breakdown during dark-induced and developmental senescence. Therefore, our results show that ppGpp signaling is not only linked to stress responses in plants but is also an important mediator of cooperation between the chloroplast and the nucleocytoplasmic compartment during plant growth and development.
© 2016 American Society of Plant Biologists. All rights reserved.
Figures




References
-
- Bang W.Y., Chen J., Jeong I.S., Kim S.W., Kim C.W., Jung H.S., Lee K.H., Kweon H.S., Yoko I., Shiina T., Bahk J.D. (2012). Functional characterization of ObgC in ribosome biogenesis during chloroplast development. Plant J. 71: 122–134. - PubMed
-
- Battesti A., Bouveret E. (2006). Acyl carrier protein/SpoT interaction, the switch linking SpoT-dependent stress response to fatty acid metabolism. Mol. Microbiol. 62: 1048–1063. - PubMed
-
- Börner T., Aleynikova A.Y., Zubo Y.O., Kusnetsov V.V. (2015). Chloroplast RNA polymerases: Role in chloroplast biogenesis. Biochim. Biophys. Acta 1847: 761–769. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

