Recommendations for the use of tolvaptan in autosomal dominant polycystic kidney disease: a position statement on behalf of the ERA-EDTA Working Groups on Inherited Kidney Disorders and European Renal Best Practice
- PMID: 26908832
- PMCID: PMC4762400
- DOI: 10.1093/ndt/gfv456
Recommendations for the use of tolvaptan in autosomal dominant polycystic kidney disease: a position statement on behalf of the ERA-EDTA Working Groups on Inherited Kidney Disorders and European Renal Best Practice
Abstract
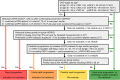
Recently, the European Medicines Agency approved the use of the vasopressin V2 receptor antagonist tolvaptan to slow the progression of cyst development and renal insufficiency of autosomal dominant polycystic kidney disease (ADPKD) in adult patients with chronic kidney disease stages 1-3 at initiation of treatment with evidence of rapidly progressing disease. In this paper, on behalf of the ERA-EDTA Working Groups of Inherited Kidney Disorders and European Renal Best Practice, we aim to provide guidance for making the decision as to which ADPKD patients to treat with tolvaptan. The present position statement includes a series of recommendations resulting in a hierarchical decision algorithm that encompasses a sequence of risk-factor assessments in a descending order of reliability. By examining the best-validated markers first, we aim to identify ADPKD patients who have documented rapid disease progression or are likely to have rapid disease progression. We believe that this procedure offers the best opportunity to select patients who are most likely to benefit from tolvaptan, thus improving the benefit-to-risk ratio and cost-effectiveness of this treatment. It is important to emphasize that the decision to initiate treatment requires the consideration of many factors besides eligibility, such as contraindications, potential adverse events, as well as patient motivation and lifestyle factors, and requires shared decision-making with the patient.
Keywords: ADPKD; tolvaptan; vasopressin V2 receptor antagonist.
© The Author 2016. Published by Oxford University Press on behalf of ERA-EDTA.
Figures
References
-
- Steinman TI. Polycystic kidney disease: a 2011 update. Curr Opin Nephrol Hypertens 2012; 21: 189–194 - PubMed
-
- Spithoven EM, Kramer A, Meijer E et al. . Analysis of data from the ERA-EDTA Registry indicates that conventional treatments for chronic kidney disease do not reduce the need for renal replacement therapy in autosomal dominant polycystic kidney disease. Kidney Int 2014; 86: 1244–1252 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

