Phosphorylation and calcium antagonistically tune myosin-binding protein C's structure and function
- PMID: 26908872
- PMCID: PMC4812749
- DOI: 10.1073/pnas.1522236113
Phosphorylation and calcium antagonistically tune myosin-binding protein C's structure and function
Abstract
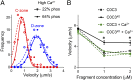
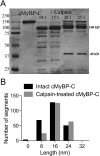
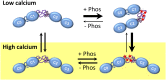
During each heartbeat, cardiac contractility results from calcium-activated sliding of actin thin filaments toward the centers of myosin thick filaments to shorten cellular length. Cardiac myosin-binding protein C (cMyBP-C) is a component of the thick filament that appears to tune these mechanochemical interactions by its N-terminal domains transiently interacting with actin and/or the myosin S2 domain, sensitizing thin filaments to calcium and governing maximal sliding velocity. Both functional mechanisms are potentially further tunable by phosphorylation of an intrinsically disordered, extensible region of cMyBP-C's N terminus, the M-domain. Using atomic force spectroscopy, electron microscopy, and mutant protein expression, we demonstrate that phosphorylation reduced the M-domain's extensibility and shifted the conformation of the N-terminal domain from an extended structure to a compact configuration. In combination with motility assay data, these structural effects of M-domain phosphorylation suggest a mechanism for diminishing the functional potency of individual cMyBP-C molecules. Interestingly, we found that calcium levels necessary to maximally activate the thin filament mitigated the structural effects of phosphorylation by increasing M-domain extensibility and shifting the phosphorylated N-terminal fragments back to the extended state, as if unphosphorylated. Functionally, the addition of calcium to the motility assays ablated the impact of phosphorylation on maximal sliding velocities, fully restoring cMyBP-C's inhibitory capacity. We conclude that M-domain phosphorylation may have its greatest effect on tuning cMyBP-C's calcium-sensitization of thin filaments at the low calcium levels between contractions. Importantly, calcium levels at the peak of contraction would allow cMyBP-C to remain a potent contractile modulator, regardless of cMyBP-C's phosphorylation state.
Keywords: cMyBP-C; muscle activation; muscle regulation; structure-function.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Comment in
-
Cardiac myosin-binding protein C: A protein once at loose ends finds its regulatory groove.Proc Natl Acad Sci U S A. 2016 Mar 22;113(12):3133-5. doi: 10.1073/pnas.1602568113. Epub 2016 Mar 10. Proc Natl Acad Sci U S A. 2016. PMID: 26966230 Free PMC article. No abstract available.
References
-
- Sequeira V, Witjas-Paalberends ER, Kuster DW, van der Velden J. Cardiac myosin-binding protein C: Hypertrophic cardiomyopathy mutations and structure-function relationships. Pflugers Arch. 2014;466(2):201–206. - PubMed
-
- Flashman E, Redwood C, Moolman-Smook J, Watkins H. Cardiac myosin binding protein C: Its role in physiology and disease. Circ Res. 2004;94(10):1279–1289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL126909/HL/NHLBI NIH HHS/United States
- R01 AR034711/AR/NIAMS NIH HHS/United States
- K99 HL124041/HL/NHLBI NIH HHS/United States
- R01 HL126909/HL/NHLBI NIH HHS/United States
- HL124041/HL/NHLBI NIH HHS/United States
- S10RR025498/RR/NCRR NIH HHS/United States
- P01 HL069779/HL/NHLBI NIH HHS/United States
- P01 HL059408/HL/NHLBI NIH HHS/United States
- R00 HL124041/HL/NHLBI NIH HHS/United States
- AR034711/AR/NIAMS NIH HHS/United States
- S10 RR025498/RR/NCRR NIH HHS/United States
- T32 HL007647/HL/NHLBI NIH HHS/United States
- HL069779/HL/NHLBI NIH HHS/United States
- T32 HL007944/HL/NHLBI NIH HHS/United States
- HL007647/HL/NHLBI NIH HHS/United States
- HL007944/HL/NHLBI NIH HHS/United States
- HL059408/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

