CXCL13 is a plasma biomarker of germinal center activity
- PMID: 26908875
- PMCID: PMC4790995
- DOI: 10.1073/pnas.1520112113
CXCL13 is a plasma biomarker of germinal center activity
Abstract
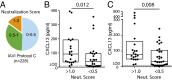
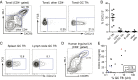
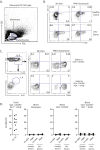
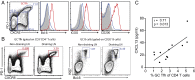
Significantly higher levels of plasma CXCL13 [chemokine (C-X-C motif) ligand 13] were associated with the generation of broadly neutralizing antibodies (bnAbs) against HIV in a large longitudinal cohort of HIV-infected individuals. Germinal centers (GCs) perform the remarkable task of optimizing B-cell Ab responses. GCs are required for almost all B-cell receptor affinity maturation and will be a critical parameter to monitor if HIV bnAbs are to be induced by vaccination. However, lymphoid tissue is rarely available from immunized humans, making the monitoring of GC activity by direct assessment of GC B cells and germinal center CD4(+) T follicular helper (GC Tfh) cells problematic. The CXCL13-CXCR5 [chemokine (C-X-C motif) receptor 5] chemokine axis plays a central role in organizing both B-cell follicles and GCs. Because GC Tfh cells can produce CXCL13, we explored the potential use of CXCL13 as a blood biomarker to indicate GC activity. In a series of studies, we found that plasma CXCL13 levels correlated with GC activity in draining lymph nodes of immunized mice, immunized macaques, and HIV-infected humans. Furthermore, plasma CXCL13 levels in immunized humans correlated with the magnitude of Ab responses and the frequency of ICOS(+) (inducible T-cell costimulator) Tfh-like cells in blood. Together, these findings support the potential use of CXCL13 as a plasma biomarker of GC activity in human vaccine trials and other clinical settings.
Keywords: CXCL13; HIV; Tfh; antibodies; vaccines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


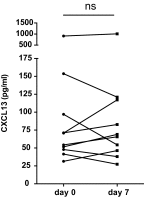

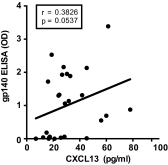
References
-
- Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. 2012;30:429–457. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

