Targeting Neural Endophenotypes of Eating Disorders with Non-invasive Brain Stimulation
- PMID: 26909013
- PMCID: PMC4754427
- DOI: 10.3389/fnins.2016.00030
Targeting Neural Endophenotypes of Eating Disorders with Non-invasive Brain Stimulation
Abstract
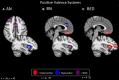
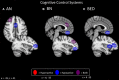
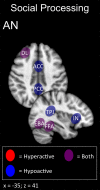
The term "eating disorders" (ED) encompasses a wide variety of disordered eating and compensatory behaviors, and so the term is associated with considerable clinical and phenotypic heterogeneity. This heterogeneity makes optimizing treatment techniques difficult. One class of treatments is non-invasive brain stimulation (NIBS). NIBS, including repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS), are accessible forms of neuromodulation that alter the cortical excitability of a target brain region. It is crucial for NIBS to be successful that the target is well selected for the patient population in question. Targets may best be selected by stepping back from conventional DSM-5 diagnostic criteria to identify neural substrates of more basic phenotypes, including behavior related to rewards and punishment, cognitive control, and social processes. These phenotypic dimensions have been recently laid out by the Research Domain Criteria (RDoC) initiative. Consequently, this review is intended to identify potential dimensions as outlined by the RDoC and the underlying behavioral and neurobiological targets associated with ED. This review will also identify candidate targets for NIBS based on these dimensions and review the available literature on rTMS and tDCS in ED. This review systematically reviews abnormal neural circuitry in ED within the RDoC framework, and also systematically reviews the available literature investigating NIBS as a treatment for ED.
Keywords: Eating Disorders (ED); RDoC; fMRI; rTMS; tDCS.
Figures


Similar articles
-
Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity.Neuroimage Clin. 2015 Mar 24;8:1-31. doi: 10.1016/j.nicl.2015.03.016. eCollection 2015. Neuroimage Clin. 2015. PMID: 26110109 Free PMC article. Review.
-
Non-invasive brain stimulation for food cravings, consumption, and disorders of eating: A review of methods, findings and controversies.Appetite. 2018 May 1;124:78-88. doi: 10.1016/j.appet.2017.03.006. Epub 2017 Mar 11. Appetite. 2018. PMID: 28288802 Review.
-
The intervention, the patient and the illness - Personalizing non-invasive brain stimulation in psychiatry.Exp Neurol. 2021 Jul;341:113713. doi: 10.1016/j.expneurol.2021.113713. Epub 2021 Mar 31. Exp Neurol. 2021. PMID: 33798562 Review.
-
Effects of non-invasive brain stimulation in children and young people with psychiatric disorders: a protocol for a systematic review.Syst Rev. 2021 Mar 11;10(1):76. doi: 10.1186/s13643-021-01627-3. Syst Rev. 2021. PMID: 33706788 Free PMC article.
-
The Effect of Non-Invasive Brain Stimulation (NIBS) on Executive Functioning, Attention and Memory in Rehabilitation Patients with Traumatic Brain Injury: A Systematic Review.Diagnostics (Basel). 2021 Mar 31;11(4):627. doi: 10.3390/diagnostics11040627. Diagnostics (Basel). 2021. PMID: 33807188 Free PMC article. Review.
Cited by
-
Neuromodulation of Eating Disorders: A Review of Underlying Neural Network Activity and Neuromodulatory Treatments.Brain Sci. 2024 Feb 22;14(3):200. doi: 10.3390/brainsci14030200. Brain Sci. 2024. PMID: 38539589 Free PMC article. Review.
-
Altered prefrontal activation during the inhibition of eating responses in women with bulimia nervosa.Psychol Med. 2023 Jun;53(8):3580-3590. doi: 10.1017/S0033291722000198. Epub 2022 Feb 25. Psychol Med. 2023. PMID: 35209961 Free PMC article.
-
Potential use of repetitive transcranial magnetic stimulation in treating pediatric avoidant/restrictive food intake disorder: a case report.Front Psychol. 2025 Jul 23;16:1593665. doi: 10.3389/fpsyg.2025.1593665. eCollection 2025. Front Psychol. 2025. PMID: 40771339 Free PMC article.
-
Clinicians' views on neuromodulation as a treatment for eating disorders: A qualitative study.Neuropsychiatr. 2021 Jun;35(2):84-91. doi: 10.1007/s40211-020-00372-8. Epub 2020 Nov 24. Neuropsychiatr. 2021. PMID: 33231833 English.
-
A pilot study exploring the effect of repetitive transcranial magnetic stimulation (rTMS) treatment on cerebral blood flow and its relation to clinical outcomes in severe enduring anorexia nervosa.J Eat Disord. 2021 Jul 9;9(1):84. doi: 10.1186/s40337-021-00420-w. J Eat Disord. 2021. PMID: 34243816 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

