Quantitative Magnetic Particle Imaging Monitors the Transplantation, Biodistribution, and Clearance of Stem Cells In Vivo
- PMID: 26909106
- PMCID: PMC4737718
- DOI: 10.7150/thno.13728
Quantitative Magnetic Particle Imaging Monitors the Transplantation, Biodistribution, and Clearance of Stem Cells In Vivo
Abstract
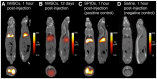
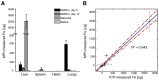
Stem cell therapies have enormous potential for treating many debilitating diseases, including heart failure, stroke and traumatic brain injury. For maximal efficacy, these therapies require targeted cell delivery to specific tissues followed by successful cell engraftment. However, targeted delivery remains an open challenge. As one example, it is common for intravenous deliveries of mesenchymal stem cells (MSCs) to become entrapped in lung microvasculature instead of the target tissue. Hence, a robust, quantitative imaging method would be essential for developing efficacious cell therapies. Here we show that Magnetic Particle Imaging (MPI), a novel technique that directly images iron-oxide nanoparticle-tagged cells, can longitudinally monitor and quantify MSC administration in vivo. MPI offers near-ideal image contrast, depth penetration, and robustness; these properties make MPI both ultra-sensitive and linearly quantitative. Here, we imaged, for the first time, the dynamic trafficking of intravenous MSC administrations using MPI. Our results indicate that labeled MSC injections are immediately entrapped in lung tissue and then clear to the liver within one day, whereas standard iron oxide particle (Resovist) injections are immediately taken up by liver and spleen. Longitudinal MPI-CT imaging also indicated a clearance half-life of MSC iron oxide labels in the liver at 4.6 days. Finally, our ex vivo MPI biodistribution measurements of iron in liver, spleen, heart, and lungs after injection showed excellent agreement (R(2) = 0.943) with measurements from induction coupled plasma spectrometry. These results demonstrate that MPI offers strong utility for noninvasively imaging and quantifying the systemic distribution of cell therapies and other therapeutic agents.
Keywords: Magnetic particle imaging; cell therapy tracking; mesenchymal stem cells; quantitative imaging.
Conflict of interest statement
Competing Interests: Drs. Conolly and Goodwill are founders and stockholders in Magnetic Insight Inc. Dr. Goodwill is an employee of the company. All other authors declare no conflicts of interest.
Figures
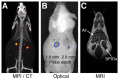

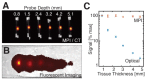
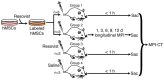
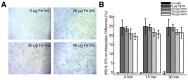


References
-
- Hoogduijn MJ, Roemeling-van Rhijn M, Engela AU, Korevaar SS, Mensah FKF, Franquesa M. et al. Mesenchymal stem cells induce an inflammatory response after intravenous infusion. Stem Cells Dev. 2013;22:2825–35. - PubMed
-
- Wu Y, Zhao RCH. The role of chemokines in mesenchymal stem cell homing to myocardium. Stem Cell Rev. 2012;8:243–50. - PubMed
-
- Ge J, Guo L, Wang S, Zhang Y, Cai T, Zhao RCH. et al. The Size of Mesenchymal Stem Cells is a Significant Cause of Vascular Obstructions and Stroke. Stem Cell Reviews and Reports. 2014;10:295–303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

