Active Tumor Permeation and Uptake of Surface Charge-Switchable Theranostic Nanoparticles for Imaging-Guided Photothermal/Chemo Combinatorial Therapy
- PMID: 26909107
- PMCID: PMC4737719
- DOI: 10.7150/thno.13686
Active Tumor Permeation and Uptake of Surface Charge-Switchable Theranostic Nanoparticles for Imaging-Guided Photothermal/Chemo Combinatorial Therapy
Erratum in
-
Erratum: Active Tumor Permeation and Uptake of Surface Charge-Switchable Theranostic Nanoparticles for Imaging-Guided Photothermal/Chemo Combinatorial Therapy: Erratum.Theranostics. 2017 Jan 7;7(3):559-560. doi: 10.7150/thno.18728. eCollection 2017. Theranostics. 2017. PMID: 28255349 Free PMC article.
Abstract
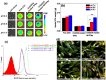
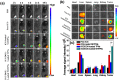
To significantly promote tumor uptake and penetration of therapeutics, a nanovehicle system comprising poly(lactic-co-glycolic acid) (PLGA) as the hydrophobic cores coated with pH-responsive N-acetyl histidine modified D-α-tocopheryl polyethylene glycol succinate (NAcHis-TPGS) is developed in this work. The nanocarriers with switchable surface charges in response to tumor extracellular acidity (pHe) were capable of selectively co-delivering indocyanine green (ICG), a photothermal agent, and doxorubicin (DOX), a chemotherapy drug, to tumor sites. The in vitro cellular uptake of ICG/DOX-loaded nanoparticles by cancer cells and macrophages was significantly promoted in weak acidic environments due to the increased protonation of the NAcHis moieties. The results of in vivo and ex vivo biodistribution studies demonstrated that upon intravenous injection the theranostic nanoparticles were substantially accumulated in TRAMP-C1 solid tumor of tumor-bearing mice. Immunohistochemical examination of tumor sections confirmed the active permeation of the nanoparticles into deep tumor hypoxia due to their small size, pHe-induced near neutral surface, and the additional hitchhiking transport via tumor-associated macrophages. The prominent imaging-guided photothermal therapy of ICG/DOX-loaded nanoparticles after tumor accumulation induced extensive tumor tissue/vessel ablation, which further promoted their extravasation and DOX tumor permeation, thus effectively suppressing tumor growth.
Keywords: chemotherapy; deep tumor penetration; photothermal therapy; surface charge transition; tumor hypoxia.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures









Similar articles
-
Chemotherapeutic drug-photothermal agent co-self-assembling nanoparticles for near-infrared fluorescence and photoacoustic dual-modal imaging-guided chemo-photothermal synergistic therapy.J Control Release. 2017 Jul 28;258:95-107. doi: 10.1016/j.jconrel.2017.05.011. Epub 2017 May 10. J Control Release. 2017. PMID: 28501673
-
NIR-Light-Triggered Anticancer Strategy for Dual-Modality Imaging-Guided Combination Therapy via a Bioinspired Hybrid PLGA Nanoplatform.Mol Pharm. 2019 Mar 4;16(3):1367-1384. doi: 10.1021/acs.molpharmaceut.8b01321. Epub 2019 Feb 22. Mol Pharm. 2019. PMID: 30776896
-
Dual Chemodrug-Loaded Single-Walled Carbon Nanohorns for Multimodal Imaging-Guided Chemo-Photothermal Therapy of Tumors and Lung Metastases.Theranostics. 2018 Feb 15;8(7):1966-1984. doi: 10.7150/thno.23848. eCollection 2018. Theranostics. 2018. PMID: 29556368 Free PMC article.
-
A Review on the Scope of Photothermal Therapy-Based Nanomedicines in Preclinical Models of Colorectal Cancer.Clin Colorectal Cancer. 2019 Jun;18(2):e200-e209. doi: 10.1016/j.clcc.2019.02.001. Epub 2019 Feb 14. Clin Colorectal Cancer. 2019. PMID: 30852125 Review.
-
Phototherapy-based combination strategies for bacterial infection treatment.Theranostics. 2020 Oct 30;10(26):12241-12262. doi: 10.7150/thno.52729. eCollection 2020. Theranostics. 2020. PMID: 33204340 Free PMC article. Review.
Cited by
-
Ceria/polymer nanocontainers for high-performance encapsulation of fluorophores.Beilstein J Nanotechnol. 2019 Feb 22;10:522-530. doi: 10.3762/bjnano.10.53. eCollection 2019. Beilstein J Nanotechnol. 2019. PMID: 30873324 Free PMC article.
-
Current Multistage Drug Delivery Systems Based on the Tumor Microenvironment.Theranostics. 2017 Jan 7;7(3):538-558. doi: 10.7150/thno.16684. eCollection 2017. Theranostics. 2017. PMID: 28255348 Free PMC article. Review.
-
Tumor Microenvironment Targeted Nanotherapy.Front Pharmacol. 2018 Oct 31;9:1230. doi: 10.3389/fphar.2018.01230. eCollection 2018. Front Pharmacol. 2018. PMID: 30429787 Free PMC article.
-
pH-responsive perylenediimide nanoparticles for cancer trimodality imaging and photothermal therapy.Theranostics. 2020 Jan 1;10(1):166-178. doi: 10.7150/thno.36999. eCollection 2020. Theranostics. 2020. PMID: 31903113 Free PMC article.
-
Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer.Chem Soc Rev. 2019 Apr 1;48(7):2053-2108. doi: 10.1039/c8cs00618k. Chem Soc Rev. 2019. PMID: 30259015 Free PMC article. Review.
References
-
- Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013;12:991–1003. - PubMed
-
- Cabral H, Nishiyama N, Kataoka K. Supramolecular nanodevices: from design validation to theranostic nanomedicine. Acc. Chem. Res. 2011;44:999–1008. - PubMed
-
- Gao GH, Li Y, Lee DS. Environmental pH-sensitive polymeric micelles for cancer diagnosis and targeted therapy. J. Controlled Release. 2013;169:180–4. - PubMed
-
- Gong H, Dong Z, Liu Y, Yin S, Cheng L, Xi W, Xiang J, Liu K, Li Y, Liu Z. Engineering of multifunctional nano-micelles for combined photothermal and photodynamic therapy under the guidance of multimodal imaging. Adv. Funct. Mater. 2014;24:6492–6502.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

