De-escalated administration of bone-targeted agents in patients with breast and prostate cancer-A survey of Canadian oncologists
- PMID: 26909274
- PMCID: PMC4723366
- DOI: 10.1016/j.jbo.2013.03.001
De-escalated administration of bone-targeted agents in patients with breast and prostate cancer-A survey of Canadian oncologists
Abstract
Objective: Questions remain regarding the optimal use of bone-targeted agents in patients with metastatic bone disease. The purpose of this study was to assess current clinical practice regarding the use and administration of bone-targeted agents by Canadian oncologists in patients with metastatic breast and prostate cancer.
Methods: A survey was designed to explore; bone-targeted agent use in metastatic bone disease, variability in the choice and the frequency of administration of these agents. Opinions were sought on potential outcomes for future trials.
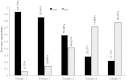
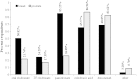
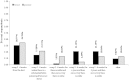
Results: A total of 193 clinicians were contacted and 90 completed our survey (response rate 49% after adjustment for inactivity). Survey respondents were medical oncologists (71.1%), radiation oncologists (21.1%) and urologists (7.8%). The findings suggest that once bone-targeted agents are started they are rarely discontinued. More agents are used in breast cancer than in prostate cancer. There was considerable interest in performing studies of de-escalated therapy in both breast and prostate cancer. Physicians requested (86%) that the primary study endpoint be the occurrence of skeletal related events and not biomarker driven.
Conclusions: Despite clinical practice guidelines and widespread use, significant areas of clinical equipoise with respect to use of bone-targeted agents exist. Findings from this survey suggest that physicians are interested in de-escalated therapy for both breast and prostate patients. However, the use of multiple agents in breast cancer and the desire for skeletal related events to be the primary endpoint means that very large randomized studies will be required.
Keywords: Bisphosphonate; Bone metastasis; Bone targeted agent; Survey.
Figures
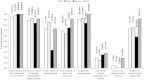
Similar articles
-
Real-world practice patterns and attitudes towards de-escalation of bone-modifying agents in patients with bone metastases from breast and prostate cancer: A physician survey.J Bone Oncol. 2020 Nov 10;26:100339. doi: 10.1016/j.jbo.2020.100339. eCollection 2021 Feb. J Bone Oncol. 2020. PMID: 33294318 Free PMC article.
-
Bone-targeted agent use for bone metastases from breast cancer and prostate cancer: A patient survey.J Bone Oncol. 2013 Jun 21;2(3):105-9. doi: 10.1016/j.jbo.2013.05.002. eCollection 2013 Sep. J Bone Oncol. 2013. PMID: 26909279 Free PMC article.
-
Adjuvant bisphosphonate use in patients with early stage breast cancer: a physician survey.Breast Cancer Res Treat. 2021 Jun;187(2):477-486. doi: 10.1007/s10549-021-06147-1. Epub 2021 Mar 23. Breast Cancer Res Treat. 2021. PMID: 33755864 Free PMC article.
-
A systematic review of dosing frequency with bone-targeted agents for patients with bone metastases from breast cancer.J Bone Oncol. 2013 May 15;2(3):123-31. doi: 10.1016/j.jbo.2013.05.001. eCollection 2013 Sep. J Bone Oncol. 2013. PMID: 26909282 Free PMC article. Review.
-
De-escalation of bone-modifying agents in patients with bone metastases from breast cancer: a systematic review and meta-analysis.Breast Cancer Res Treat. 2019 Aug;176(3):507-517. doi: 10.1007/s10549-019-05265-1. Epub 2019 May 11. Breast Cancer Res Treat. 2019. PMID: 31079283
Cited by
-
The Rethinking Clinical Trials (REaCT) Program. A Canadian-Led Pragmatic Trials Program: Strategies for Integrating Knowledge Users into Trial Design.Curr Oncol. 2021 Oct 4;28(5):3959-3977. doi: 10.3390/curroncol28050337. Curr Oncol. 2021. PMID: 34677255 Free PMC article. Review.
-
Perceptions around bone-modifying agent use in patients with bone metastases from breast and castration resistant prostate cancer: a patient survey.Support Care Cancer. 2021 Nov;29(11):6903-6912. doi: 10.1007/s00520-021-06238-1. Epub 2021 May 22. Support Care Cancer. 2021. PMID: 34023950 Free PMC article.
-
Two-year results of a randomised trial comparing 4- versus 12-weekly bone-targeted agent use in patients with bone metastases from breast or castration-resistant prostate cancer.J Bone Oncol. 2021 Sep 2;30:100388. doi: 10.1016/j.jbo.2021.100388. eCollection 2021 Oct. J Bone Oncol. 2021. PMID: 34567960 Free PMC article.
-
Bone-Targeted Agents for the Management of Breast Cancer Patients with Bone Metastases.J Clin Med. 2013 Aug 19;2(3):67-88. doi: 10.3390/jcm2030067. J Clin Med. 2013. PMID: 26237063 Free PMC article. Review.
-
Third-party online surveys-science, selling, or sugging?Curr Oncol. 2015 Jun;22(3):182-3. doi: 10.3747/co.22.2448. Curr Oncol. 2015. PMID: 26089716 Free PMC article. No abstract available.
References
-
- Bouganim N., Dranitsaris G., Amir E, Clemons M. Optimising the use of bone-targeted agents in patients with metastatic cancers: a practical guide for medical oncologists. Support Care Center. 2011;19:1687–1698. - PubMed
-
- Hortobagyi G., Theriault R., Porter L., Blayney D, Lipton A, Sinoff C. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. Protocal 19 Aredia Breast Cancer Study Group. New England Journal of Medicine. 1996;335:1785–1791. - PubMed
-
- Thiebaud D., Jaeger P., Jacquet A., Burchkardt P. Dose-response in the treatment of hypercalcemis of malignancy by a single infusion of the bisphosphonate AHPrBP. Journal of Clinical Oncology. 1988;6:762–768. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

