Ataxin-10 is part of a cachexokine cocktail triggering cardiac metabolic dysfunction in cancer cachexia
- PMID: 26909315
- PMCID: PMC4735665
- DOI: 10.1016/j.molmet.2015.11.004
Ataxin-10 is part of a cachexokine cocktail triggering cardiac metabolic dysfunction in cancer cachexia
Erratum in
-
'Corrigendum to "Ataxin-10 is part of a cachexokine cocktail triggering cardiac metabolic dysfunction in cancer cachexia" [Molecular Metabolism 5 (2) (2015) 67-78]'.Mol Metab. 2020 May;35:100970. doi: 10.1016/j.molmet.2020.02.013. Epub 2020 Mar 4. Mol Metab. 2020. PMID: 32244184 Free PMC article. No abstract available.
Abstract
Objectives: Cancer cachexia affects the majority of tumor patients and significantly contributes to high mortality rates in these subjects. Despite its clinical importance, the identity of tumor-borne signals and their impact on specific peripheral organ systems, particularly the heart, remain mostly unknown.
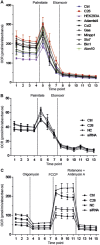
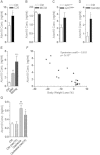
Methods and results: By combining differential colon cancer cell secretome profiling with large-scale cardiomyocyte phenotyping, we identified a signature panel of seven "cachexokines", including Bridging integrator 1, Syntaxin 7, Multiple inositol-polyphosphate phosphatase 1, Glucosidase alpha acid, Chemokine ligand 2, Adamts like 4, and Ataxin-10, which were both sufficient and necessary to trigger cardiac atrophy and aberrant fatty acid metabolism in cardiomyocytes. As a prototypical example, engineered secretion of Ataxin-10 from non-cachexia-inducing cells was sufficient to induce cachexia phenotypes in cardiomyocytes, correlating with elevated Ataxin-10 serum levels in murine and human cancer cachexia models.
Conclusions: As Ataxin-10 serum levels were also found to be elevated in human cachectic cancer patients, the identification of Ataxin-10 as part of a cachexokine cocktail now provides a rational approach towards personalized predictive, diagnostic and therapeutic measures in cancer cachexia.
Keywords: Ataxin-10; Cancer cachexia; Cardiac dysfunction; Fatty acid metabolism.
Figures

References
-
- Fearon K., Arends J., Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nature Reviews Clinical Oncology. 2013;10:90–99. - PubMed
-
- Dewys W.D., Begg C., Lavin P.T., Band P.R., Bennett J.M., Bertino J.R. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. American Journal of Medicine. 1980;69:491–497. - PubMed
-
- Tisdale M.J. Cachexia in cancer patients. Nature Reviews Cancer. 2002;2:862–871. - PubMed
-
- Martignoni M.E., Kunze P., Hildebrandt W., Kunzli B., Berberat P., Giese T. Role of mononuclear cells and inflammatory cytokines in pancreatic cancer-related cachexia. Clinical Cancer Research. 2005;11:5802–5808. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

