Mutant Kras copy number defines metabolic reprogramming and therapeutic susceptibilities
- PMID: 26909577
- PMCID: PMC4780242
- DOI: 10.1038/nature16967
Mutant Kras copy number defines metabolic reprogramming and therapeutic susceptibilities
Abstract
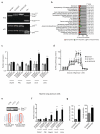
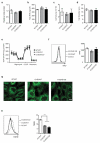
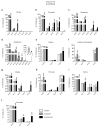
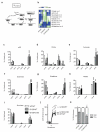
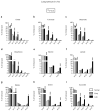
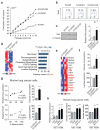
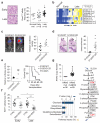
The RAS/MAPK (mitogen-activated protein kinase) signalling pathway is frequently deregulated in non-small-cell lung cancer, often through KRAS activating mutations. A single endogenous mutant Kras allele is sufficient to promote lung tumour formation in mice but malignant progression requires additional genetic alterations. We recently showed that advanced lung tumours from Kras(G12D/+);p53-null mice frequently exhibit Kras(G12D) allelic enrichment (Kras(G12D)/Kras(wild-type) > 1) (ref. 7), implying that mutant Kras copy gains are positively selected during progression. Here we show, through a comprehensive analysis of mutant Kras homozygous and heterozygous mouse embryonic fibroblasts and lung cancer cells, that these genotypes are phenotypically distinct. In particular, Kras(G12D/G12D) cells exhibit a glycolytic switch coupled to increased channelling of glucose-derived metabolites into the tricarboxylic acid cycle and glutathione biosynthesis, resulting in enhanced glutathione-mediated detoxification. This metabolic rewiring is recapitulated in mutant KRAS homozygous non-small-cell lung cancer cells and in vivo, in spontaneous advanced murine lung tumours (which display a high frequency of Kras(G12D) copy gain), but not in the corresponding early tumours (Kras(G12D) heterozygous). Finally, we demonstrate that mutant Kras copy gain creates unique metabolic dependences that can be exploited to selectively target these aggressive mutant Kras tumours. Our data demonstrate that mutant Kras lung tumours are not a single disease but rather a heterogeneous group comprising two classes of tumours with distinct metabolic profiles, prognosis and therapeutic susceptibility, which can be discriminated on the basis of their relative mutant allelic content. We also provide the first, to our knowledge, in vivo evidence of metabolic rewiring during lung cancer malignant progression.
Figures




Comment in
-
Targeting metabolic peculiarities: a potentially novel therapeutic approach for KRAS-mutant lung cancer?J Thorac Dis. 2016 Aug;8(8):E794-5. doi: 10.21037/jtd.2016.06.72. J Thorac Dis. 2016. PMID: 27618791 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

