Antimicrobial blue light inactivation of Candida albicans: In vitro and in vivo studies
- PMID: 26909654
- PMCID: PMC5026794
- DOI: 10.1080/21505594.2016.1155015
Antimicrobial blue light inactivation of Candida albicans: In vitro and in vivo studies
Abstract
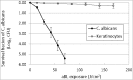
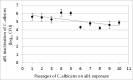
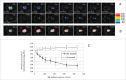
Fungal infections are a common cause of morbidity, mortality and cost in critical care populations. The increasing emergence of antimicrobial resistance necessitates the development of new therapeutic approaches for fungal infections. In the present study, we investigated the effectiveness of an innovative approach, antimicrobial blue light (aBL), for inactivation of Candida albicans in vitro and in infected mouse burns. A bioluminescent strain of C. albicans was used. The susceptibilities to aBL (415 nm) were compared between C. albicans and human keratinocytes. The potential development of aBL resistance by C. albicans was investigated via 10 serial passages of C. albicans on aBL exposure. For the animal study, a mouse model of thermal burn infected with the bioluminescent C. albicans strain was used. aBL was delivered to mouse burns approximately 12 h after fungal inoculation. Bioluminescence imaging was performed to monitor in real time the extent of infection in mice. The results obtained from the studies demonstrated that C. albicans was approximately 42-fold more susceptible to aBL than human keratinocytes. Serial passaging of C. albicans on aBL exposure implied a tendency of reduced aBL susceptibility of C. albicans with increasing numbers of passages; however, no statistically significant difference was observed in the post-aBL survival rate of C. albicans between the first and the last passage (P>0.05). A single exposure of 432 J/cm(2) aBL reduced the fungal burden in infected mouse burns by 1.75-log10 (P=0.015). Taken together, our findings suggest aBL is a potential therapeutic for C. albicans infections.
Keywords: antimicrobial blue light; bioluminescence imagining; burn; candida albicans; endogenous photosensitizer; mouse model.
Figures



Comment in
-
New insights into the antimicrobial blue light inactivation of Candida albicans.Virulence. 2016 Jul 3;7(5):493-4. doi: 10.1080/21505594.2016.1160194. Epub 2016 Mar 7. Virulence. 2016. PMID: 26950053 Free PMC article. No abstract available.
References
-
- d'Enfert C. Hidden killers: persistence of opportunistic fungal pathogens in the human host. Curr Opin Microbiol 2009; 12:358-64; PMID:19541532; http://dx.doi.org/ 10.1016/j.mib.2009.05.008 - DOI - PubMed
-
- Shoham S, Marwaha S. Invasive fungal infections in the ICU. J Intensive Care Med 2010; 25:78-92; PMID:19955115; http://dx.doi.org/ 10.1177/0885066609355262 - DOI - PubMed
-
- Ballard J, Edelman L, Saffle J, Sheridan R, Kagan R, Bracco D, Cancio L, Cairns B, Baker R, Fillari P, et al.. Positive fungal cultures in burn patients: a multicenter review. J Burn Care Res 2008; 29:213-21; PMID:18182925 - PubMed
-
- Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Sci Transl Med 2012; 4:165rv13; PMID:23253612; http://dx.doi.org/ 10.1126/scitranslmed.3004404 - DOI - PubMed
-
- Vicente MF, Basilio A, Cabello A, Pelaez F. Microbial natural products as a source of antifungals. Clin Microbiol Infect 2003; 9:15-32; PMID:12691539; http://dx.doi.org/ 10.1046/j.1469-0691.2003.00489.x - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
