Calreticulin Regulates Neointima Formation and Collagen Deposition following Carotid Artery Ligation
- PMID: 26910059
- PMCID: PMC4816666
- DOI: 10.1159/000443884
Calreticulin Regulates Neointima Formation and Collagen Deposition following Carotid Artery Ligation
Abstract
Background/aims: The endoplasmic reticulum (ER) stress protein, calreticulin (CRT), is required for the production of TGF-β-stimulated extracellular matrix (ECM) by fibroblasts. Since TGF-β regulates vascular fibroproliferative responses and collagen deposition, we investigated the effects of CRT knockdown on vascular smooth-muscle cell (VSMC) fibroproliferative responses and collagen deposition.
Methods: Using a carotid artery ligation model of vascular injury, Cre-recombinase-IRES-GFP plasmid was delivered with microbubbles (MB) to CRT-floxed mice using ultrasound (US) to specifically reduce CRT expression in the carotid artery.
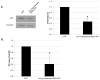
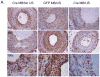
Results: In vitro, Cre-recombinase-mediated CRT knockdown in isolated, floxed VSMCs decreased the CRT transcript and protein, and attenuated the induction of collagen I protein in response to TGF-β. TGF-β stimulation of collagen I was partly blocked by the NFAT inhibitor 11R-VIVIT. Following carotid artery ligation, CRT staining was upregulated with enhanced expression in the neointima 14-21 days after injury. Furthermore, Cre-recombinase-IRES-GFP plasmid delivered by targeted US reduced CRT expression in the neointima of CRT-floxed mice and led to a significant reduction in neointima formation and collagen deposition. The neointimal cell number was also reduced in mice, with a local, tissue-specific knockdown of CRT.
Conclusions: This work establishes a novel role for CRT in mediating VSMC responses to injury through the regulation of collagen deposition and neointima formation.
© 2016 S. Karger AG, Basel.
Conflict of interest statement
Figures










Similar articles
-
MFAP4 Promotes Vascular Smooth Muscle Migration, Proliferation and Accelerates Neointima Formation.Arterioscler Thromb Vasc Biol. 2016 Jan;36(1):122-33. doi: 10.1161/ATVBAHA.115.306672. Epub 2015 Nov 12. Arterioscler Thromb Vasc Biol. 2016. PMID: 26564819
-
Mindin regulates vascular smooth muscle cell phenotype and prevents neointima formation.Clin Sci (Lond). 2015 Jul;129(2):129-45. doi: 10.1042/CS20140679. Clin Sci (Lond). 2015. PMID: 25751394
-
The Ca(v)3.1 T-type calcium channel is required for neointimal formation in response to vascular injury in mice.Cardiovasc Res. 2012 Dec 1;96(3):533-42. doi: 10.1093/cvr/cvs257. Epub 2012 Aug 10. Cardiovasc Res. 2012. PMID: 22886848
-
Inhibition of Smooth Muscle β-Catenin Hinders Neointima Formation After Vascular Injury.Arterioscler Thromb Vasc Biol. 2017 May;37(5):879-888. doi: 10.1161/ATVBAHA.116.308643. Epub 2017 Mar 16. Arterioscler Thromb Vasc Biol. 2017. PMID: 28302627 Free PMC article.
-
The role of the endoplasmic reticulum protein calreticulin in mediating TGF-β-stimulated extracellular matrix production in fibrotic disease.J Cell Commun Signal. 2018 Mar;12(1):289-299. doi: 10.1007/s12079-017-0426-2. Epub 2017 Oct 28. J Cell Commun Signal. 2018. PMID: 29080087 Free PMC article. Review.
Cited by
-
Disease-Relevant Single Cell Photonic Signatures Identify S100β Stem Cells and their Myogenic Progeny in Vascular Lesions.Stem Cell Rev Rep. 2021 Oct;17(5):1713-1740. doi: 10.1007/s12015-021-10125-x. Epub 2021 Mar 17. Stem Cell Rev Rep. 2021. PMID: 33730327 Free PMC article.
-
Calreticulin is important for the development of renal fibrosis and dysfunction in diabetic nephropathy.Matrix Biol Plus. 2020 Apr 3;8:100034. doi: 10.1016/j.mbplus.2020.100034. eCollection 2020 Nov. Matrix Biol Plus. 2020. PMID: 33543033 Free PMC article.
-
Calreticulin and cancer.Cell Res. 2021 Jan;31(1):5-16. doi: 10.1038/s41422-020-0383-9. Epub 2020 Jul 30. Cell Res. 2021. PMID: 32733014 Free PMC article. Review.
-
Screening key miRNAs and genes in prostate cancer by microarray analysis.Transl Cancer Res. 2020 Feb;9(2):856-868. doi: 10.21037/tcr.2019.12.30. Transl Cancer Res. 2020. PMID: 35117431 Free PMC article.
-
Endoplasmic reticulum in health and disease: the 12th International Calreticulin Workshop, Delphi, Greece.J Cell Mol Med. 2017 Dec;21(12):3141-3149. doi: 10.1111/jcmm.13413. Epub 2017 Nov 21. J Cell Mol Med. 2017. PMID: 29160038 Free PMC article.
References
-
- Hoffmann R, Mintz GS, Dussaillant GR, Popma JJ, Pichard AD, Satler LF, Kent KM, Griffin J, Leon MB. Patterns and mechanisms of in-stent restenosis. A serial intravascular ultrasound study. Circulation. 1996;94:1247–1254. - PubMed
-
- Chaabane C, Otsuka F, Virmani R, Bochaton-Piallat ML. Biological responses in stented arteries. Cardiovasc Res. 2013;99:353–363. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

