Biomarker-Guided Adaptive Trial Designs in Phase II and Phase III: A Methodological Review
- PMID: 26910238
- PMCID: PMC4766245
- DOI: 10.1371/journal.pone.0149803
Biomarker-Guided Adaptive Trial Designs in Phase II and Phase III: A Methodological Review
Abstract
Background: Personalized medicine is a growing area of research which aims to tailor the treatment given to a patient according to one or more personal characteristics. These characteristics can be demographic such as age or gender, or biological such as a genetic or other biomarker. Prior to utilizing a patient's biomarker information in clinical practice, robust testing in terms of analytical validity, clinical validity and clinical utility is necessary. A number of clinical trial designs have been proposed for testing a biomarker's clinical utility, including Phase II and Phase III clinical trials which aim to test the effectiveness of a biomarker-guided approach to treatment; these designs can be broadly classified into adaptive and non-adaptive. While adaptive designs allow planned modifications based on accumulating information during a trial, non-adaptive designs are typically simpler but less flexible.
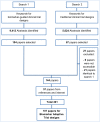
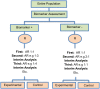
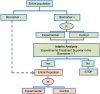
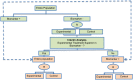
Methods and findings: We have undertaken a comprehensive review of biomarker-guided adaptive trial designs proposed in the past decade. We have identified eight distinct biomarker-guided adaptive designs and nine variations from 107 studies. Substantial variability has been observed in terms of how trial designs are described and particularly in the terminology used by different authors. We have graphically displayed the current biomarker-guided adaptive trial designs and summarised the characteristics of each design.
Conclusions: Our in-depth overview provides future researchers with clarity in definition, methodology and terminology for biomarker-guided adaptive trial designs.
Conflict of interest statement
Figures

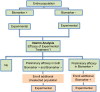
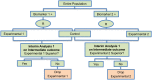
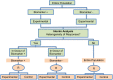
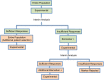
References
-
- Chabner B. Advances and challenges in the use of biomarkers in clinical trials. Clinical advances in hematology & oncology: H&O. 2008;6(1):42–3. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

